Abstract
Background. Granulocytic sarcoma (GS), or myeloid sarcoma or chloroma, is a tumoral mass containing myeloblasts and immature granulocytes in an anatomic site other than the bone marrow. GS is very rare in children with acute promyelocytic leukemia (APL). This case report presents a rare case of GS manifesting as a solitary bone mass.
Case. A 15-year-old female presented with left knee pain. Complete blood count and biochemistry were normal. No blasts or early granulocytic elements were observed in the peripheral blood smear. Magnetic resonance imaging (MRI) revealed a 4x4-cm solid lesion extending to the physis line in the distal metaphyseal section of the left femur. A Tru-cut biopsy of the mass confirmed GS with immature promyelocytic cell infiltration containing Auer rods and immature myeloid cells. The t(15;17) mutation was highly positive in the tissue suspension. Bone marrow aspiration performed afterward showed no abnormalities, and acute myeloid leukemia and acute lymphoblastic leukemia mutation panels were negative. The patient was diagnosed as having APL presenting as GS of isolated femoral origin. Treatment with standard-risk chemotherapy, including all-trans retinoic acid (ATRA) according to the BFM 2013 protocol, was initiated. After 2 months, a repeat biopsy showed no pathologic promyelocytic infiltration and a negative t(15;17) mutation. However, the patient died of severe neutropenia, sepsis, and typhoid fever.
Conclusion. This case contributes to the literature as a rare presentation of APL as isolated femoral GS. It is the first reported case of an isolated femoral mass in this context to the best of our knowledge.
Keywords: granulocytic sarcoma, myeloid sarcoma, chloroma, acute promyelocytic leukemia, neutropenia
Introduction
Granulocytic sarcoma (GS), also known as myeloid sarcoma or chloroma, is defined as the appearance of a tumoral mass containing myeloblasts and immature granulocytes in an anatomic site other than the bone marrow1 GS can occur before, concurrently, or after a diagnosis of acute myeloblastic leukemia (AML). It may develop as a relapse in the setting of myeloproliferative diseases such as myelodysplastic syndrome, chronic myeloid leukemia, or even after achieving remission with AML treatment.1 APL occurs in about 7% of children with AML. APL is a distinct subtype of AML.2 In children with acute promyelocytic leukemia (APL), GS is rare and typically involves tissues such as skin, gingiva, central nervous system, lung, mediastinal lymph nodes, and testis in relapse cases.3,4 This case report presents a rare case of GS manifesting as a solitary bone mass.
Case Presentation
A 15-year-old female patient presented with a 3-week history of left knee pain and swelling. Her medical history revealed no specific signs. She had no fever on admission, and her vital signs were stable. A firm, slightly tender mass approximately 4x5 cm in size, located 5 cm proximal to the left knee joint was observed after physical examination. No redness or temperature increase was observed in the mass. No organomegaly or lymphadenopathy was observed.
A complete blood count revealed the following: white blood cells (WBC), 4800/mm³; neutrophils, 2800/mm³; hemoglobin, 14.4 g/dL; and platelets, 303,000/mm³. A peripheral blood smear showed 60% neutrophils, 30% lymphocytes, and 10% monocytes. Erythrocytes were normochromic and normocytic, platelets were abundant and clustered, and no blasts were observed.
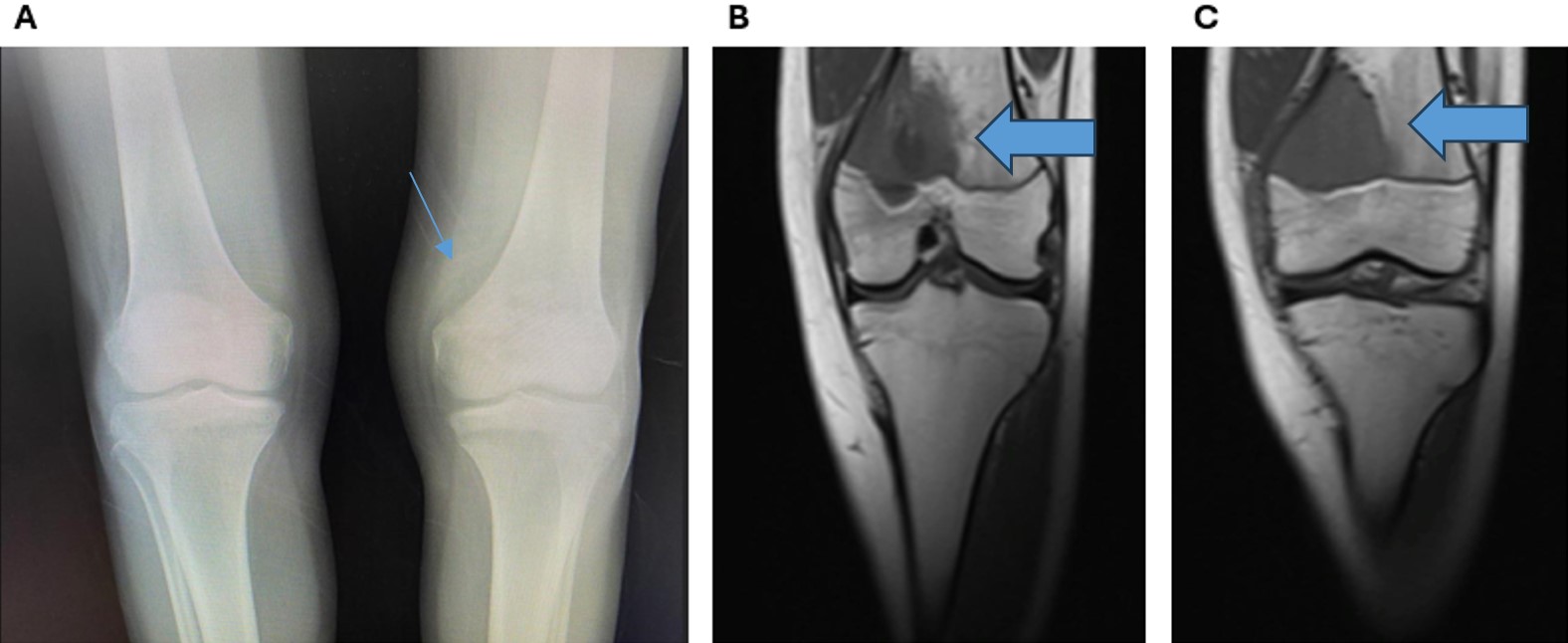
The chest X-ray and abdominal ultrasonography were normal. Direct radiography showed millimetric lytic lesions medial to the distal metaphysis of the left femur with a surrounding lamellar periosteal reaction (Fig. 1). The mass was evaluated to be a primary bone tumor. Magnetic resonance imaging (MRI) of the left knee revealed a 4x4-cm solid lesion extending to the physis-line in the distal metaphyseal section of the left femur with medial cortical destruction. The mass extended into the soft tissue (Fig. 2).

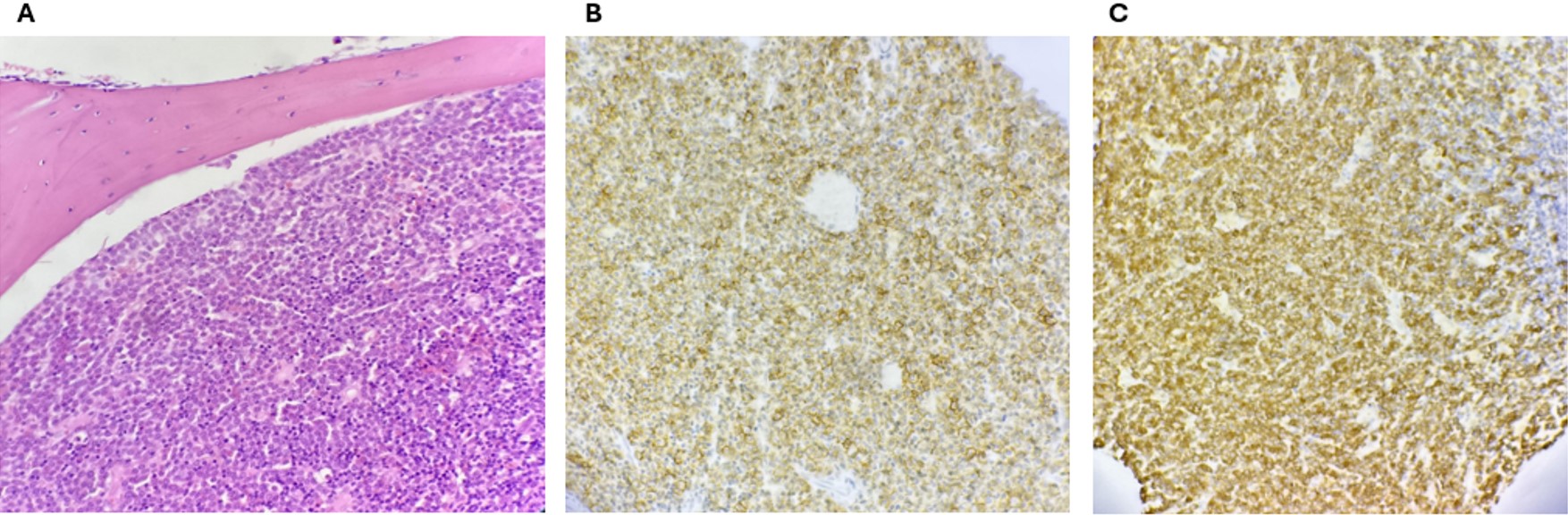
A Tru-cut biopsy of the femoral bone mass was performed under general anesthesia as the first diagnostic step. A pathology examination confirmed GS with immature promyelocytic cell infiltration containing Auer rods and immature myeloid cells (Fig. 3). Pathologic promyelocytes containing Auer rods were observed in the imprint smear prepared from the Tru-cut biopsy material. The t(15;17) translocation mutation was highly positive in the tissue suspension prepared from the biopsy material. Based on these findings, the patient was diagnosed as having APL, presenting as GS of isolated femoral origin.
To eliminate systemic involvement, bone marrow aspiration and biopsy were performed from the pelvic bone marrow. Standard bone marrow smears demonstrated no systemic myeloid disease; repeated biopsies and aspirations from this site revealed a regular cellular pattern without any malignancy. The t(15;17) mutation was also negative in the bone marrow aspirate.
A standard chemotherapy protocol, including all-trans-retinoic acid (ATRA) treatments according to the AML Berlin-Frankfurt-Munich (BFM) 2013 guideline, was initiated. An ATRA regimen was administered 3 days prior to intravenous (IV) chemotherapy because the WBC count was lower than 10,000/mm³. The protocol consisted of single induction therapy with anthracycline (daunorubicin or idarubicin) plus cytarabine, followed by three courses of consolidation therapy. MRI showed no significant change in the mass size 2 months after chemotherapy. Therefore, another Tru-cut biopsy was performed. The pathology examination revealed no pathologic promyelocytic infiltration and a negative t(15;17) mutation. Based on the biopsy results, the treatment was thought to be beneficial and continuation of chemotherapy along with surgical resection of the mass was planned.
The patient received AIE (cytarabine, idarubicin, etoposide) and AI (cytarabine, idarubicin) chemotherapy blocks as per the protocol. Following these blocks, the patient experienced deep and prolonged cytopenias, neutropenic fever, and feeding problems. Unfortunately, the patient died of severe neutropenia, sepsis, and septic shock after the second block of chemotherapy. Informed consent was obtained from the patient’s family for the publication of this case report.
Discussion
The prognosis for pediatric AML has improved, and the long-term survival rate has now approached 70%. Approximately 95% of children with APL achieve a complete response (CR), and the event-free survival (EFS) and overall survival (OS) rates are 80-90% and approximately 90%, respectively. Patients often present with fatal disseminated intravascular coagulation (DIC) in induction chemotherapy. Chloromal involvement is seen in 2-5% of patients with childhood AML, typically in AML M2, M4, and M5 subtypes, regardless of sex.1,5 Therapy restricted to local procedures, even those that appear to be cured by resection or irradiation, increases the risk of systemic disease. When left untreated, most cases of primary GS progress to overt leukemia.6
Worch et al.4 reported a similar case. Their patient was a 16-year-old boy with lesions on the femur, tibia, and humerus. However, reverse transcription polymerase chain reaction (RT-PCR) demonstrated the presence of t(15;17) PML/RARα fusion mRNA from the pelvis in both peripheral blood and bone marrow. We were unsuccessful in investigating t(15;17) using fluorescence in situ hybridization (FISH) only in pelvic bone marrow. Accordingly, we recommend using RT-PCR to investigate t(15;17) in bone marrow. Shimizu et al.7 retrospectively analyzed 434 consecutive patients with AML. Forty-five (10.4%) patients with GS at diagnosis were younger and were more likely to conform to the French–American–British M4 and M5 classifications than those without GS. The site of GS was bone in four cases (8.9%), and one was APL. Complete remission rates did not differ significantly between the GS and non-GS groups. The GS group had a significantly higher relapse rate than the non-GS group and a significantly lower 5-year disease-free survival (DFS) rate. However, acute promyelocytic leukemia was excluded from the survival analysis. Thirty-nine (15%) patients achieved CR after the second induction therapy. Although Shimizu et al.’s7 study was conducted on adult patients, it may also provide a guide for child patients because of the large patient sample. Patients did not achieve CR after the first induction therapy but did so after the second, like our patient.
Harrer et al.8 reported a 67-year-old male patient with APL presenting with a tumor in the right piriform sinus, accompanied by a mini-review of 16 similar cases of extramedullary APL manifestations. Among these, three cases involved bone lesions, specifically located in the vertebrae, sternum, and shoulder. These cases provide additional context for understanding the variability of extramedullary APL presentations. In the patient with a sternal lesion, blood counts were normal, coagulopathy was absent, and bone marrow was not affected. In contrast, the patient with a vertebral lesion exhibited leukopenia, absent coagulopathy, and t(15;17) was identified in the bone marrow. The patient with a shoulder lesion had anemia; although coagulopathy was not mentioned, t(15;17) was also detected in the bone marrow. In contrast to these cases, our patient presented with a femoral mass, normal blood count, no evidence of coagulopathy, and no detectable t(15;17) in the bone marrow. This combination of findings is exceptionally rare and adds to the diversity of extramedullary APL presentations. The absence of coagulopathy and normal hematologic parameters in our patient highlights the diagnostic challenges in identifying APL in such atypical scenarios.8
The early use of ATRA can improve prognosis, regardless of whether sarcoma is the first or a recurrent manifestation in APL. A misdiagnosis of lymphoma may occur, and diagnostic distinction can be difficult in the isolated presentation of myeloid sarcoma without any signs of leukemia.4,9 Tissue examination plays a very important role in the diagnosis because some patients have no bone marrow involvement at the time of onset. When fresh tissue samples cannot be obtained, cytogenetic abnormalities of fixed and paraffin embedded sections can be detected using FISH.10 When the PML/RARα fusion gene is found, it is recommended to use ATRA treatment and monitor the condition through peripheral blood and bone marrow.
The optimum therapy is also unclear. Wang et al.11 published a review of cases, reporting that 20 APL patients with myeloid sarcoma received ATRA with chemotherapy; 16 achieved CR, two achieved partial remission (PR), and the other two died of sepsis and cerebral hemorrhage. In the same review, eight patients were treated with chemotherapy only without ATRA or ATO; two achieved CR, one achieved PR, and the remaining five died. The longest follow-up was 96 months. In this case, the patient was treated with radiotherapy + ATRA + chemotherapy, although radiotherapy or tumor resection together with ATRA and chemotherapy may improve the prognosis of myeloid sarcoma (MS)/APL. MS/APL has diverse clinical manifestations, molecular biology, and cytogenetics, is easily confused with stromal tumor, lymphoma, and carcinoma, and is therefore associated with a high misdiagnosis rate, poor prognosis, and high recurrence rate.11
There is limited information about APL with GS in the literature; therefore, our case presentation makes a valuable contribution to existing knowledge. However, given the isolated nature of the bone mass, it was thought that this treatment regimen alone would be insufficient. Therefore, ATRA treatment was combined with standard-risk chemotherapy per the AML BFM 2013 protocol. Evaluating remission solely based on morphologic and cytogenetic features was because the bone marrow was not leukemic. In our case, the MRI performed after AIE and AI blocks showed no significant change in the mass size. Conversely, the biopsy revealed extensive necrosis within the mass and no tumoral tissue. Therefore, relying solely on mass size reduction to assess treatment response in patients with GS, similar to soft tissue sarcomas, may be misleading. The plan was to continue chemotherapy without additional radiotherapy and consider a bone graft if needed. Unfortunately, the patient died of neutropenic sepsis and septic shock. Although similar outcomes are observed with classic APL treatment in patients with GS, OS and DFS are significantly lower in these cases. This suggests the need for alternative treatment strategies specifically tailored for patients with GS.1,7
Conclusion
This case report underscores the rare presentation of APL as an isolated femoral GS without classic hematologic abnormalities, coagulopathy, or detectable t(15;17) in the bone marrow. This atypical case adds to the spectrum of APL manifestations in pediatric patients and highlights the diagnostic challenges associated with such presentations. Further research is essential to optimize treatment strategies and improve outcomes for rare extramedullary APL cases.
Ethical approval
Written informed consent was obtained from the patient’s family.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res 2013; 3: 265-270.
- PDQ Pediatric Treatment Editorial Board. Childhood Acute Promyelocytic Leukemia Treatment (PDQ®): Health Professional Version. In: PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); June 14, 2024.
- Yamashita Y, Isomura N, Hamasaki Y, Goto M. Case of pediatric acute promyelocytic leukemia presenting as extramedullary tumor of the mandible. Head Neck 2013; 35: E310-E313. https://doi.org/10.1002/hed.23163
- Worch J, Ritter J, Frühwald MC. Presentation of acute promyelocytic leukemia as granulocytic sarcoma. Pediatr Blood Cancer 2008; 50: 657-660. https://doi.org/10.1002/pbc.21190
- Magdy M, Abdel Karim N, Eldessouki I, Gaber O, Rahouma M, Ghareeb M. Myeloid sarcoma. Oncol Res Treat 2019; 42: 224-229. https://doi.org/10.1159/000497210
- Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer 2002; 94: 1739-1746. https://doi.org/10.1002/cncr.10399
- Shimizu H, Saitoh T, Hatsumi N, et al. Clinical significance of granulocytic sarcoma in adult patients with acute myeloid leukemia. Cancer Sci 2012; 103: 1513-1517. https://doi.org/10.1111/j.1349-7006.2012.02324.x
- Harrer DC, Lüke F, Einspieler I, et al. Case report: extramedullary acute promyelocytic leukemia: an unusual case and mini-review of the literature. Front Oncol 2022; 12: 886436. https://doi.org/10.3389/fonc.2022.886436
- Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology 1999; 34: 391-398. https://doi.org/10.1046/j.1365-2559.1999.00651.x
- Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007; 21: 340-350. https://doi.org/10.1038/sj.leu.2404491
- Wang L, Cai DL, Lin N. Myeloid sarcoma of the colon as initial presentation in acute promyelocytic leukemia: a case report and review of the literature. World J Clin Cases 2021; 9: 6017-6025. https://doi.org/10.12998/wjcc.v9.i21.6017
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















