Graphical Abstract
Abstract
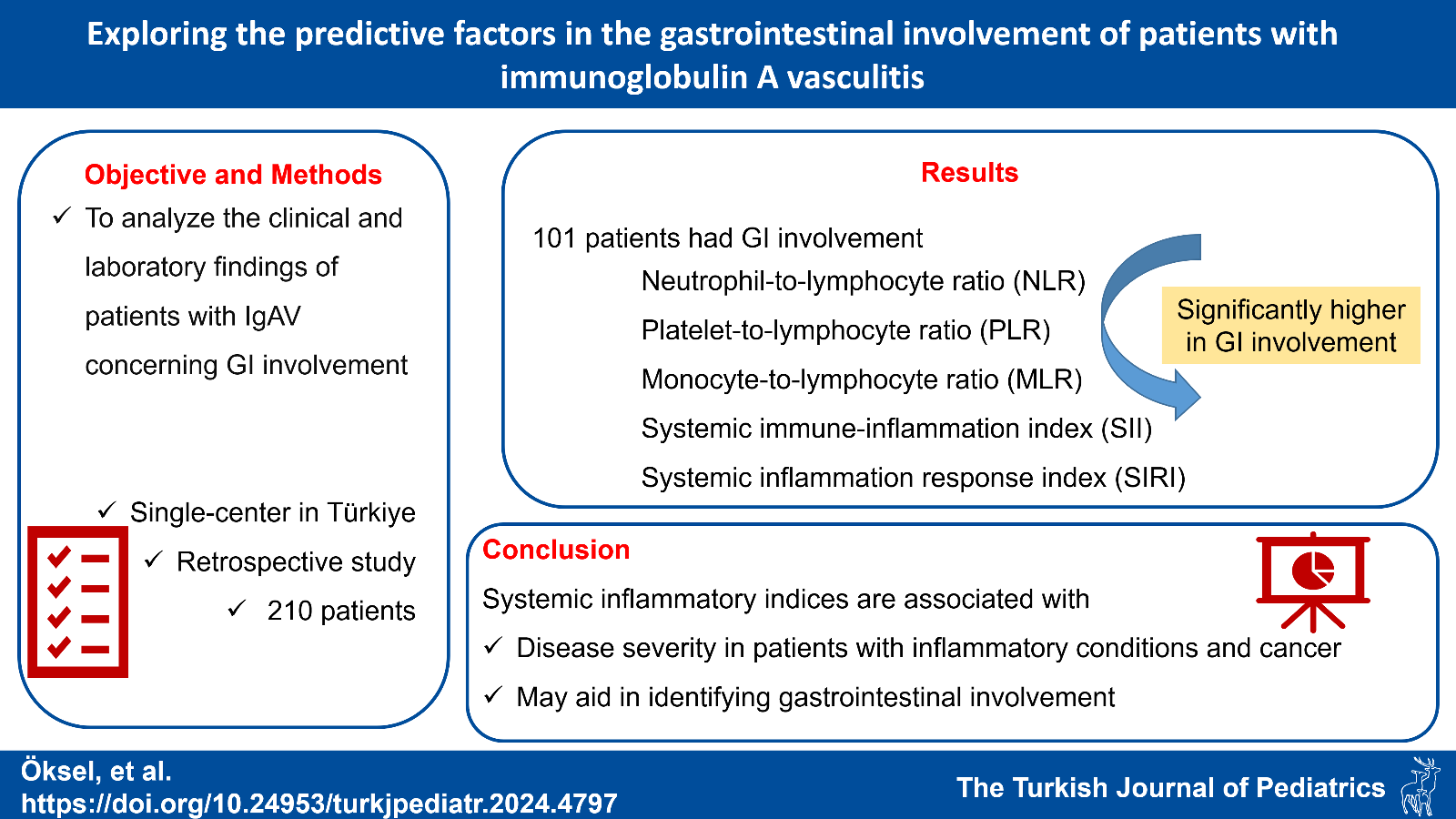
Background. Immunoglobulin A vasculitis (IgAV), the most common systemic vasculitis in children, typically presents with gastrointestinal (GI) symptoms in about half of cases. This study aimed to analyze the clinical and laboratory findings of patients with IgAV regarding GI involvement.
Methods. We compared the GI involvement data of the patients diagnosed with IgAV.
Results. Of the 210 patients (60.5% female and 39.5% male), 101 had GI involvement, with abdominal pain being the predominant symptom (n=98). White blood cell, neutrophil, monocyte, and platelet counts, C-reactive protein, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), systemic immune-inflammation index (SII), and systemic inflammation response index (SIRI) were significantly elevated in patients with GI involvement (p<0.001, p<0.001, p=0.01, p=0.005, p=0.002, p<0.001, p=0.03, p=0.001, p<0.001, p<0.001, respectively). The cutoff values for SII (>1035.7), SIRI (>1.65), NLR (>2.73), and MLR (>0.28) were determined, yielding respective sensitivities of 46%, 59%, 47%, and 53%, specificities of 83.1%, 69.1%, 81.3%, and 71.9%. Corresponding areas under the curve were 0.658, 0.668, 0.649, and 0.634, respectively (all p<0.001).
Conclusion. Although IgAV is a self-limiting disease, GI involvement can lead to serious consequences. Systemic inflammatory indices such as SII and SIRI may be indicative in identifying patients with GI involvement.
Keywords: immunoglobulin A vasculitis, gastrointestinal involvement, Henoch-Schönlein purpura
References
- Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum 2005; 35: 143-153. https://doi.org/10.1016/j.semarthrit.2005.08.007
- Yang YH, Hung CF, Hsu CR, et al. A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein purpura in Taiwan. Rheumatology (Oxford) 2005; 44: 618-622. https://doi.org/10.1093/rheumatology/keh544
- Oni L, Sampath S. Childhood IgA vasculitis (Henoch Schonlein Purpura)-advances and knowledge gaps. Front Pediatr 2019; 7: 257. https://doi.org/10.3389/fped.2019.00257
- Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: final classification criteria. Ann Rheum Dis 2010; 69: 798-806. https://doi.org/10.1136/ard.2009.116657
- Chang WL, Yang YH, Lin YT, Chiang BL. Gastrointestinal manifestations in Henoch-Schönlein purpura: a review of 261 patients. Acta Paediatr 2004; 93: 1427-1431. https://doi.org/10.1080/08035250410020181
- Makay B, Gücenmez ÖA, Duman M, Ünsal E. The relationship of neutrophil-to-lymphocyte ratio with gastrointestinal bleeding in Henoch-Schonlein purpura. Rheumatol Int 2014; 34: 1323-1327. https://doi.org/10.1007/s00296-014-2986-2
- Hong SH, Kim CJ, Yang EM. Neutrophil-to-lymphocyte ratio to predict gastrointestinal bleeding in Henoch: Schönlein purpura. Pediatr Int 2018; 60: 791-795. https://doi.org/10.1111/ped.13652
- Karadağ ŞG, Çakmak F, Çil B, et al. The relevance of practical laboratory markers in predicting gastrointestinal and renal involvement in children with Henoch-Schönlein Purpura. Postgrad Med 2021; 133: 272-277. https://doi.org/10.1080/00325481.2020.1807161
- Lee LE, Pyo JY, Ahn SS, Song JJ, Park YB, Lee SW. Systemic inflammation response index predicts all-cause mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Int Urol Nephrol 2021; 53: 1631-1638. https://doi.org/10.1007/s11255-020-02777-4
- Huang T, Peng Q, Zhang Y, Zhu Z, Fan X. The Systemic Immune-Inflammation Index (SII) and coronary artery lesions in Kawasaki disease. Clin Exp Med 2024; 24: 4. https://doi.org/10.1007/s10238-023-01265-0
- Niculescu R, Russu E, Arbănași EM, et al. Carotid plaque features and inflammatory biomarkers as predictors of restenosis and mortality following carotid endarterectomy. Int J Environ Res Public Health 2022; 19: 13934. https://doi.org/10.3390/ijerph192113934
- Russu E, Mureșan AV, Arbănași EM, et al. The predictive role of NLR and PLR in outcome and patency of lower limb revascularization in patients with femoropopliteal disease. J Clin Med 2022; 11: 2620. https://doi.org/10.3390/jcm11092620
- Lei WT, Tsai PL, Chu SH, et al. Incidence and risk factors for recurrent Henoch-Schönlein purpura in children from a 16-year nationwide database. Pediatr Rheumatol Online J 2018; 16: 25. https://doi.org/10.1186/s12969-018-0247-8
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360: 1197-1202. https://doi.org/10.1016/S0140-6736(02)11279-7
- Calviño MC, Llorca J, García-Porrúa C, Fernández-Iglesias JL, Rodriguez-Ledo P, González-Gay MA. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore) 2001; 80: 279-290. https://doi.org/10.1097/00005792-200109000-00001
- Shim JO, Han K, Park S, Kim GH, Ko JS, Chung JY. Ten-year nationwide population-based survey on the characteristics of children with Henoch-Schönlein purpura in Korea. J Korean Med Sci 2018; 33: e174. https://doi.org/10.3346/jkms.2018.33.e174
- Hwang HH, Lim IS, Choi BS, Yi DY. Analysis of seasonal tendencies in pediatric Henoch-Schönlein purpura and comparison with outbreak of infectious diseases. Medicine (Baltimore) 2018; 97: e12217. https://doi.org/10.1097/MD.0000000000012217
- Wang S, Tang H, Du W, Ding Y. Massive gastrointestinal hemorrhage caused by Henoch-Schoenlein purpura: a case report. Medicine (Baltimore) 2021; 100: e28240. https://doi.org/10.1097/MD.0000000000028240
- Jauhola O, Ronkainen J, Koskimies O, et al. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: a 6-month prospective study. Arch Dis Child 2010; 95: 871-876. https://doi.org/10.1136/adc.2009.167874
- Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam study: a population-based prospective cohort study. Sci Rep 2018; 8: 10566. https://doi.org/10.1038/s41598-018-28646-w
- Gayret OB, Erol M, Tekin Nacaroglu H. The relationship of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio with gastrointestinal bleeding in Henoch-Schonlein purpura. Iran J Pediatr 2016; 26: e8191. https://doi.org/10.5812/ijp.8191
- Yakut HI, Kurt T, Uncu N, Semsa Cayci F, Celikel Acar B. Predictive role of neutrophil to lymphocyte ratio and mean platelet volume in Henoch-Schönlein purpura related gastrointestinal and renal involvement. Arch Argent Pediatr 2020; 118: 139-142. https://doi.org/10.5546/aap.2020.eng.139
- Suszek D, Górak A, Majdan M. Differential approach to peripheral blood cell ratios in patients with systemic lupus erythematosus and various manifestations. Rheumatol Int 2020; 40: 1625-1629. https://doi.org/10.1007/s00296-020-04669-3
- Seringec Akkececi N, Yildirim Cetin G, Gogebakan H, Acipayam C. The C-reactive protein/albumin ratio and complete blood count parameters as indicators of disease activity in patients with Takayasu arteritis. Med Sci Monit 2019; 25: 1401-1409. https://doi.org/10.12659/MSM.912495
- Du J, Chen S, Shi J, et al. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol 2017; 36: 2689-2695. https://doi.org/10.1007/s10067-017-3815-2
- Yuan Y, Liu J, Zhou Y, et al. The relationship between monocyte-to-lymphocyte ratio and the risk of gastrointestinal system involvement in children with IgA vasculitis: a preliminary report. Adv Clin Exp Med 2021; 30: 999-1005. https://doi.org/10.17219/acem/138906
- Li Y, Feng X, Huang L, et al. Hematologic and immunological characteristics of Henoch-Schönlein purpura in rat and rabbit models induced with ovalbumin based on type III hypersensitivity. Sci Rep 2015; 5: 8862. https://doi.org/10.1038/srep08862
Copyright and license
Copyright © 2024 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















