Graphical Abstract
Abstract
Background. Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by epidermal skin barrier dysfunction and altered immune response. Electrical impedance spectroscopy (EIS) has been used as a novel tool to detect skin barrier changes in AD. EIS is a non-invasive measure of the electrical impedance of tissue and is sensitive to cellular structure and extracellular environment.
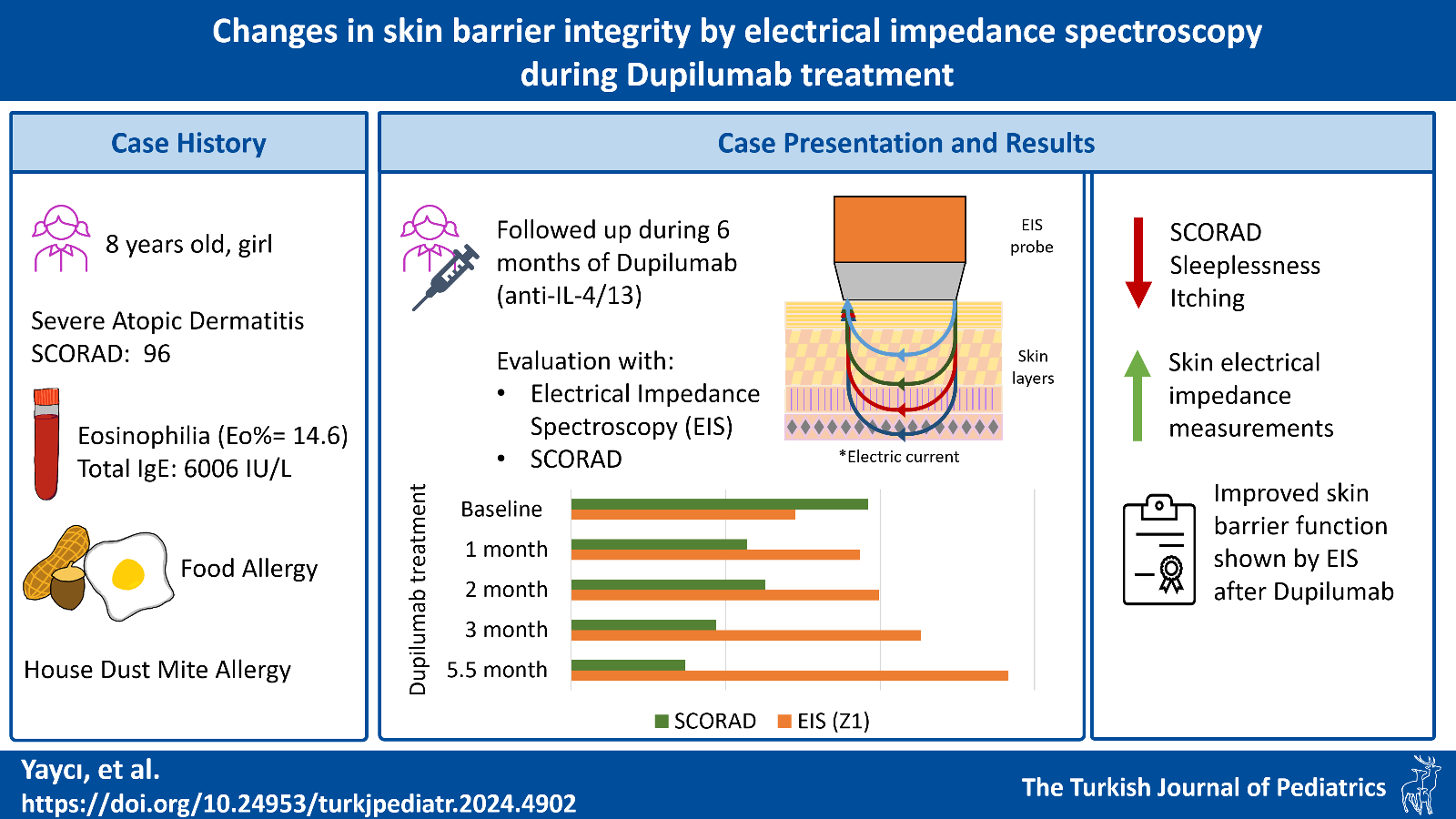
Case Presentation. An 8-year-old girl presented with severe AD, starting at 3 years of age. She also had allergic rhinitis, food allergies, and sensitization to mites, eggs, and nuts. Unresponsive to other treatments, she was administered 300 mg of dupilumab, a monoclonal antibody inhibiting IL-4 and IL-13 activity. Patient’s response to the treatment and skin barrier integrity was followed for 6 months: First at the baseline (before dupilumab) and then again at the 1st, 2nd, 3rd, and 5.5th month after dupilumab with SCORing Atopic Dermatitis (SCORAD), as well as measurements of moisture by MoistureMeterSC (Delfin®) and EIS by Nevisense® (SciBase) on the forearm and antecubital fossa of the same arm. At the end of 6 months, her SCORAD improved from 96 to 37. The moisture measurements were variable. The EIS by Z1 score in the forearm increased from 72 to 141 and EIS by MIX scores increased from 2.7 to 6.2. The correlation between SCORAD and forearm EIS by Z1 and MIX scores were significant: r=-0.913, (p=0.03) and r=-0.881, (p=0.049). The correlation between forearm MIX scores with sleeplessness and itching was significant: r=-0.956, (p=0.011), r=-0.942, (p=0.017).
Conclusion. As higher EIS scores reflect stronger barrier integrity, the increase in Z1 and MIX obtained from Nevisense® implies an improvement in the skin barrier integrity during dupilumab treatment. This report highlights the potential use of EIS in atopic dermatitis patients to evaluate treatment efficacy. We urge rapid and non-invasive use of EIS in pediatrics to be further investigated in clinical settings.
Keywords: atopic dermatitis, children, electric impedance spectroscopy, epithelium, dupilumab
References
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers 2018; 4: 1. https://doi.org/10.1038/s41572-018-0001-z
- Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy 2015; 45: 566-574. https://doi.org/10.1111/cea.12495
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol 2017; 1027: 21-37. https://doi.org/10.1007/978-3-319-64804-0_3
- Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy 2020; 50: 5-14. https://doi.org/10.1111/cea.13491
- Berdyshev E, Goleva E, Bissonnette R, et al. Dupilumab significantly improves skin barrier function in patients with moderate-to-severe atopic dermatitis. Allergy 2022; 77: 3388-3397. https://doi.org/10.1111/all.15432
- Rinaldi AO, Korsfeldt A, Ward S, et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy 2021; 76: 3066-3079. https://doi.org/10.1111/all.14842
- Sasaki M, Sundberg M, Frei R, et al. Electrical impedance spectroscopy detects skin barrier dysfunction in childhood atopic dermatitis. Allergy 2024; 79: 142-152. https://doi.org/10.1111/all.15895
- Rinaldi AO, Morita H, Wawrzyniak P, et al. Direct assessment of skin epithelial barrier by electrical impedance spectroscopy. Allergy 2019; 74: 1934-1944. https://doi.org/10.1111/all.13824
- SciBase. Nevisense Clinical Reference Guide. 2014. Article Number: 975-0009-04. Available at: https://scibase.com/wp-content/uploads/2017/11/Clinical-Reference-Guide-1.pdf
- Huygen L, Thys PM, Wollenberg A, Gutermuth J, Krohn IK. Skin barrier function assessment: electrical impedance spectroscopy is less influenced by daily routine activities than transepidermal water loss. Ann Dermatol 2024; 36: 99-111. https://doi.org/10.5021/ad.23.052
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial [published correction appears in J Am Acad Dermatol 2021; 84: 230]. J Am Acad Dermatol 2020; 83: 1282-1293. https://doi.org/10.1016/j.jaad.2020.06.054
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab safety and efficacy in a phase III open-label extension trial in children 6-11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb) 2023; 13: 2697-2719. https://doi.org/10.1007/s13555-023-01016-9
- Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143: 155-172. https://doi.org/10.1016/j.jaci.2018.08.022
- de Bruin Weller MS, Knulst AC, Meijer Y, Bruijnzeel-Koomen CAFM, Pasmans SGM. Evaluation of the child with atopic dermatitis. Clin Exp Allergy 2012; 42: 352-362. https://doi.org/10.1111/j.1365-2222.2011.03899.x
- Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol 2007; 157: 645-648. https://doi.org/10.1111/j.1365-2133.2007.08112.x
Copyright and license
Copyright © 2024 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















