Abstract
Background. Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a fatal, genetically transmitted cardiomyopathy that can cause unpredictable malignant life-threatening arrhythmias. Arrhythmias that may be hemodynamically insignificant in healthy persons, yet may be fatal in patients with cardiomyopathy and end-stage heart failure. Thus, urgent and prompt management of arrhythmias in these patients is essential to achieve favorable outcomes.
Case Presentation. Here, we present a 13-year-old male who was referred to our institution with a prediagnosis of ARVC and had sudden cardiac arrest on the second day due to ventricular tachycardia / fibrillation. Successful extracorporeal cardiopulmonary resuscitation (E-CPR) was performed. A successful endo-epicardial ablation of ventricular tachycardia and implantable cardiac defibrillator insertion were performed under extracorporeal membrane oxygenation (ECMO) due to recurrent malignant ventricular arrhythmias. On the fourth day, he was weaned from ECMO without any sequelae. Although the patient did not experience any hemodynamically significant or sustained tachycardia after catheter ablation, he underwent a successful transplantation due to progressive heart failure.
Conclusion. Appropriate and urgent management of life-threatening arrhythmias and when necessary high-quality resuscitation measures including E-CPR and a multidisciplinary coordinated approach is crucial in the management of patients with cardiomyopathy and end-stage heart failure.
Keywords: arrhythmogenic right ventricular cardiomyopathy, implantable cardiac defibrillator, ablation, transplantation
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited disorder characterized by progressive fibro-fatty replacement of the myocardium, and ventricular tachycardia (VT) with left bundle branch block (LBBB) morphology. It usually presents with symptomatic arrhythmias or sudden death.1
Diagnosis of arrhythmogenic cardiomyopathy is made using revised Padua criteria (2020) that are based on a multi parametric approach encompassing functional and structural ventricular abnormalities, tissue characterization findings, depolarization and repolarization alterations, ventricular arrhythmias and familial/genetic background.2 The most striking clinical features that must alert physicians are signs and symptoms of right heart failure, VT originating from the right ventricle, negative T waves and epsilon waves in leads V1-V3. Other diagnostic tools include electrocardigraphy, echocardiography, magnetic resonance imaging (MRI), and genetic tests.
Several concurrent processes have been proposed as responsible for the arrhythmogenicity in ARVC, including triggered activity linked to sympathetic activity, re-entrant mechanisms caused by myocardial fibrosis, myocardial inflammation, and/or ion channel failure.3,4 These variables could account for the occurrence of both hemodynamically stable monomorphic VT and rapid, unstable rhythms such as polymorphic VT or ventricular fibrillation in patients with ARVC.5-7 Roudijk et al. reported that male sex, the quantity of T-wave inversions in the precordial leads, ventricular ectopy on Holter monitoring, and a lower biventricular ejection fraction on cardiac imaging are all linked to arrhythmic occurrences.7
Here, a 13-year-old male diagnosed with ARVC, who experienced sudden cardiac arrest due to malignant ventricular arrhythmia (VA) and successfully bridged to heart transplantation is presented.
Case presentation
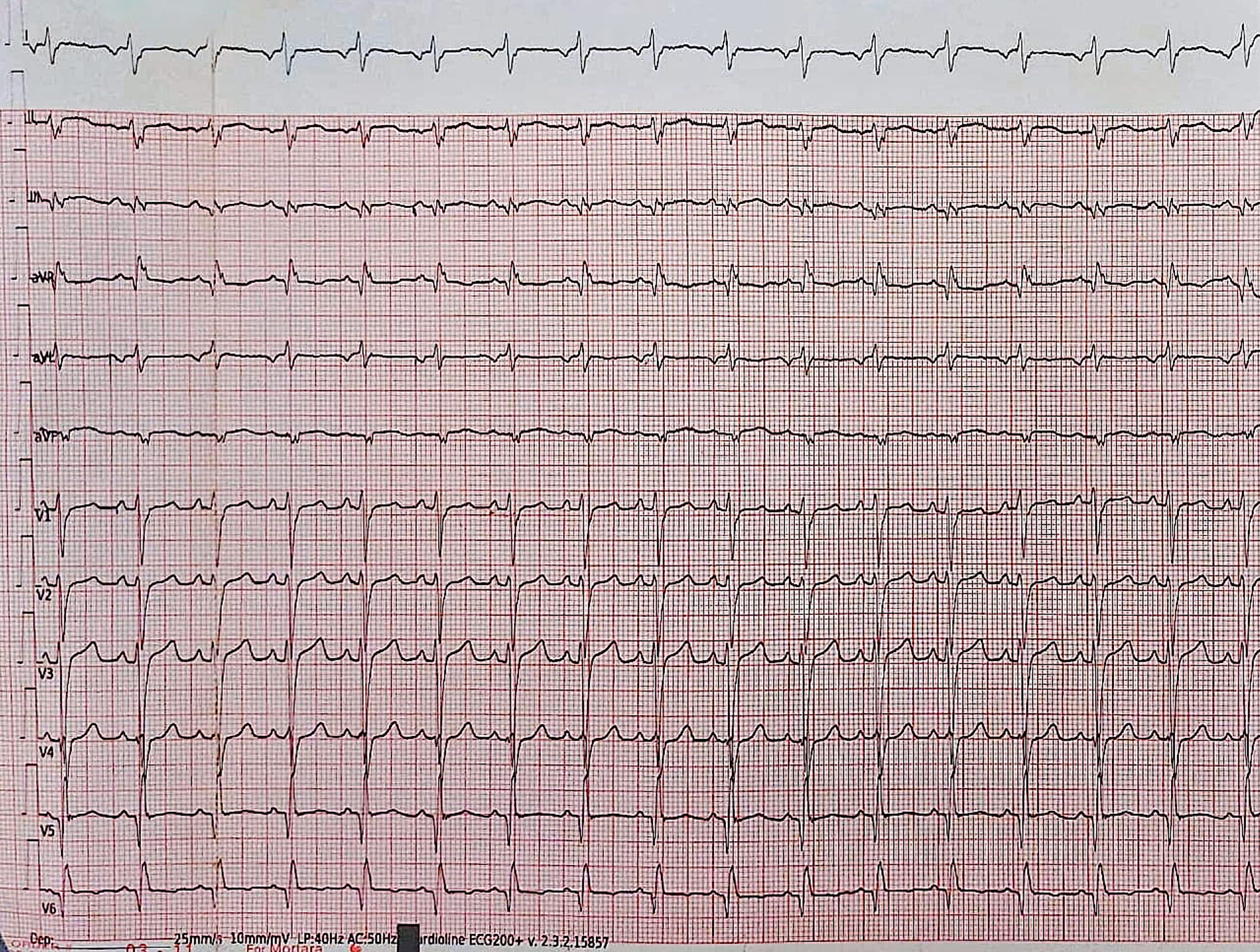
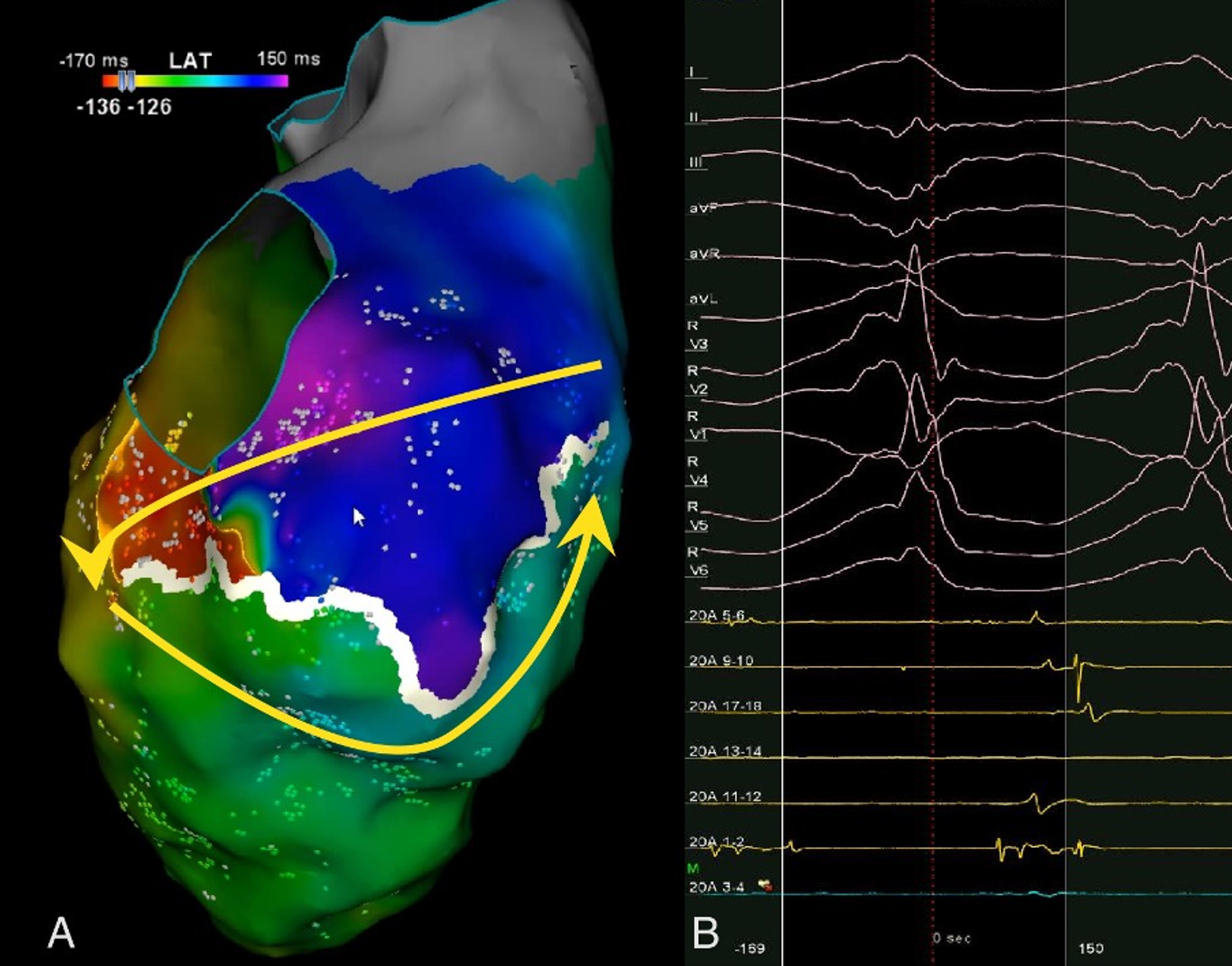
A 13-year-old male patient was admitted with complaints of chest pain and shortness of breath with exertion for 2 months. He was referred to our institution with a prediagnosis of ARVC and heart failure. Physical examination findings were as follows: Mild tachycardia (120-130 bpm), tachypnea (38/min), a 2-3/6 pansystolic murmur best heard at the left lower sternal border, jugular venous distension, a liver palpable 3 cm below the costal margin and mild ascites. Electrocardiogram showed; sinus tachycardia (110 bpm), a widened QRS (120 msec) and the corrected QT interval (QTc) of 480 msec (because of widened QRS) (Fig. 1). Echocardiography revealed significantly reduced biventricular function (left ventricle M-mode ejection fraction: 15%), enlarged right heart chambers with a RV diameter of 53.8 mm (z score: + 4.08) in apical 4 chamber view and severe tricuspid regurgitation (Fig. 2). Cardiac MRI showed marked dilation of the right ventricle (RV) with an indexed volume of 149 mL/m2 and hypokinesia in the free and inferior walls, as well as segmental wall thinning and focal late gadolinium enhancement (Fig. 3). The ejection fraction was 10% and 15% for the right and left ventricles, respectively. Heart failure treatment was administered immediately and a 24-hour-rhythm Holter was planned. On the second day of hospitalization, the patient had a sudden cardiac arrest and during cardiopulmonary resuscitation, it was recognized that the patient had VT exhibiting LBBB morphology with an intermittent transition to ventricular fibrillation (VF). Multiple antiarrhythmics including lidocaine, amiodarone, and magnesium were administered and the arrhythmia was converted to sinus rhythm after 5 cardioversions and 2 defibrillations. The patient was transferred to the pediatric intensive care unit (PICU). During follow-up in the PICU, the patient had episodes of LBBB morphology VT with intermittent transition to VF despite amiodarone and lidocaine infusion and was re-arrested. During cardiopulmonary resuscitation (CPR), venoarterial extracorporeal membrane oxygenation (ECMO) was initiated in 35 minutes. As drug-resistant VT storm continued during ECMO support, a single staged endocardial and epicardial approach for ablation was planned along with the assistance of cardiothoracic surgery. Even though the epicardial scar was much larger than the endocardial scar, a clinical VT isthmus was present at the endocardial site where ablation terminated the VT quickly (Fig. 4). An implantable cardiac defibrillator was implanted for secondary prevention, amiodarone was administered, and the patient was decannulated on the 4th day. The patient was discharged without any sequelae meanwhile autosomal dominant mutation that is linked to ARVC was detected in DSC2 gene at 18q12.1. Three months later the patient was rehospitalized for decompensated heart failure (NYHA class III-IV). Following this, his clinical status deteriorated due to multidrug refractory and inotrope-dependent heart failure. His kidney functions worsened, he became symptomatic even at rest, and developed massive hepatomegaly and ascites which required multiple paracentesis but never experienced sustained and hemodynamically significant arrhythmia. He could not be discharged and was on inotropes for 12 months till he underwent a successful heart transplantation. Pathological analysis of the explanted heart revealed: diffuse degenerative changes characterized by nuclear hyperchromasia, centralization and pleomorphism, areas of mucinous degeneration and oedema under the endocardium, mucinous degeneration in the valves, fibrosis between muscle fibers, congestion and fibrin accumulation sites in the pericardium and also a quite thin right ventricle wall. The patient has been followed up for 9 months and shown no symptoms post-transplantation.
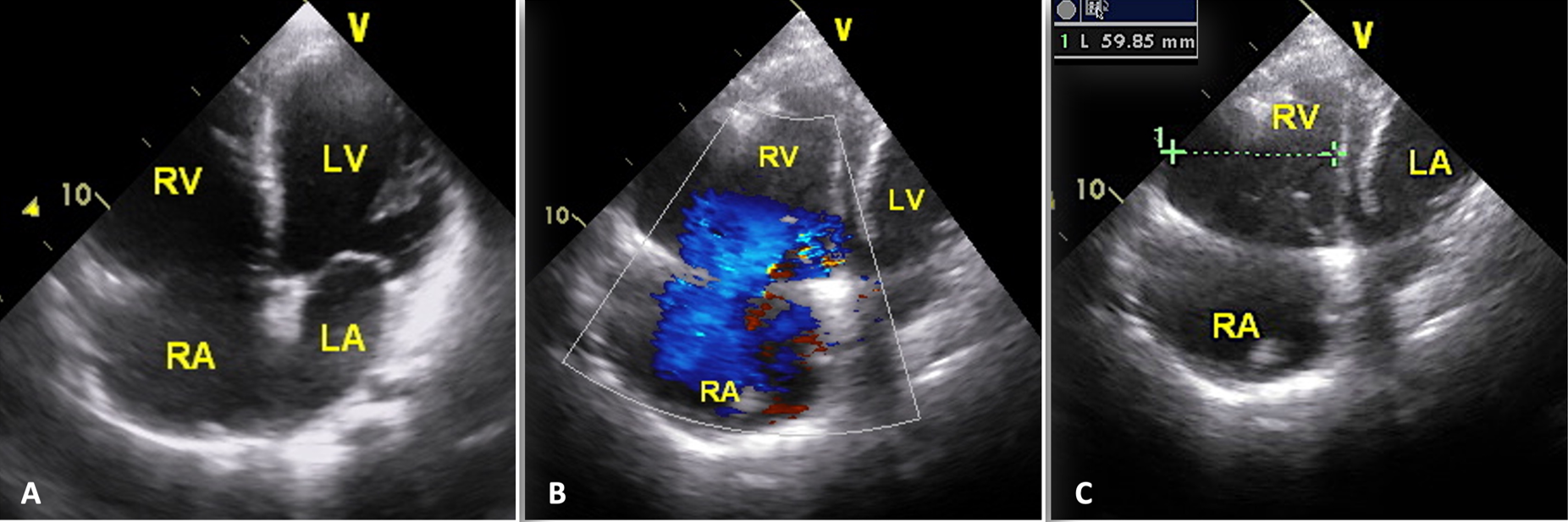
B: severe tricuspid regurgitation; C: apical 4-chamber view with RV dimension.
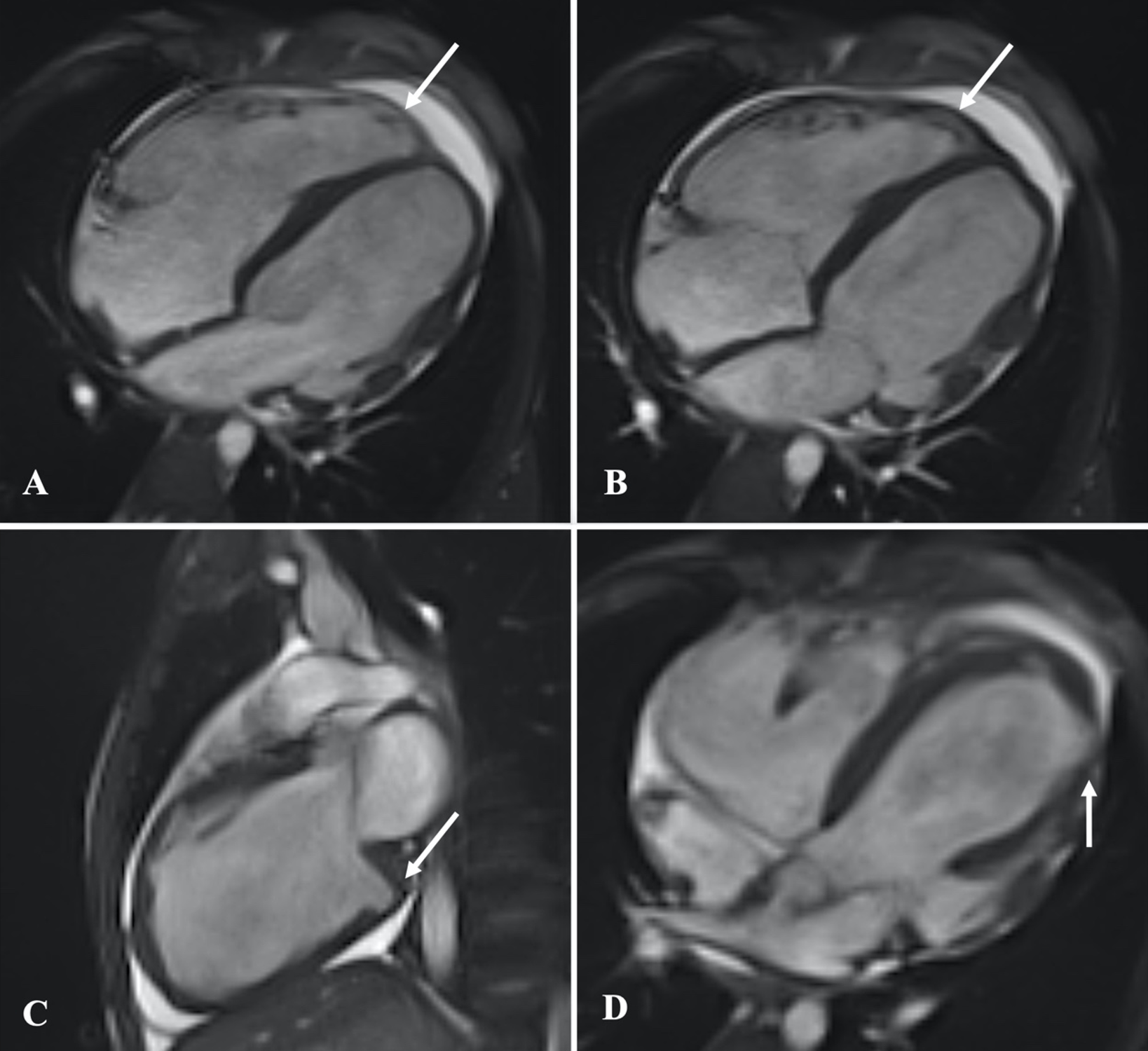
The patient’s family gave their informed consent for this case report to be published.
Discussion
A case of ARVC who had sudden cardiac arrest due to VT and successfully bridged to heart transplantation is presented here. We aimed to emphasize life-threatening arrhythmias and sudden cardiac arrest that may occur in patients with cardiomyopathy and end-stage heart failure (HF), as well as the prompt management of these patients with high-quality resuscitation measures and a multidisciplinary coordinated approach to achieve favorable outcomes.
Our patient fulfilled the diagnosis of ARVC according to the Padau criteria and had experienced sudden cardiac arrest (SCA) due to VT / VF in the absence of a prior history of syncope and palpitation. Consistent with the literature, as a risk factor for life-threatening arrhythmias, our patient was male and had biventricular failure.
The main goals of treatment are to prevent SCA, slow the rate of disease progression and reduce VA. In patients with cardiomyopathy and heart failure, malignant arrhythmias are the first cause to rule out in case of rapid clinical deterioration or sudden cardiac arrest as in our patient. Although implantable cardioverter – defibrillators (ICDs) can be used as primary or secondary prevention in these patients, they are not beneficial and may cause electrical storms in the presence of uncontrolled ventricular arrhythmias. In cases experiencing VA in the presence of appropriate medical treatment catheter ablation of VA may be necessary in addition to ICD implantation.8,9 Even though traditional endocardial ablation is quite effective, some patients do not respond well to ablation due to the existence of epicardial reentrant circuits. Up to 30% of the substrates of aberrant ventricular activity are intramural or subepicardial.10 Pokushalov et al. reported that epicardial ablation may be necessary and that it increases overall success in adolescent patients with ARVC in whom endocardial ablation of VT has failed.11 Several recent publications indicate that simultaneous epicardial and endocardial ablation is superior to endocardial ablation alone in means of VA recurrence without a significant difference in all-cause mortality or acute procedural complications and may even result in the permanent elimination of this arrhythmia.12
Epicardial catheter ablation has been demonstrated to be safe and effective in adults, but there are limited reports in the pediatric population.13 Both endocardial and epicardial ablation may be challenging in hemodynamically unstable patients. In infants with incessant tachyarrhythmias, extracorporeal membrane oxygenation provides a hemodynamically stable and safe platform for antiarrhythmic drug therapy and ablation.13 Our patient was already under ECMO support because of aborted cardiac arrest and was still clinically deteriorating due to multidrug refractory VT / VF storm. At this point, ablation under ECMO was our only option. Similarly, Thomas et al performed epicardial ablation in a 13-month-old infant and reported that venoarterial ECMO provides hemodynamic stability in the face of unstable arrhythmias. In cases with hemodynamic instability, ablation may be performed with ECMO support to achieve hemodynamic stability.
Our patient who suffered SCA was successfully discharged from the hospital without any sequelae after prompt treatment of arrhythmia with epi-endo mapping and endocardial ablation, ICD implantation and amiodarone treatment. He had never had clinically significant or sustained arrhythmia up until he underwent a successful heart transplantation due to refractory heart failure. As in our patient, in cases with refractory heart failure, the sole treatment option is heart transplantation.14
In cases with uncontrollable life-threatening arrhythmias, E-CPR and ECMO may bridge to successful recovery and transplantation.
In conclusion, malignant life-threatening arrhythmias and sudden cardiac arrest may occur in patients with ARVC. High-quality resuscitation measures and a multidisciplinary coordinated approach including E-CPR and ECMO play a crucial role and have a life-saving potential for survival during in-hospital sudden cardiac arrest. Successful endo/epicardial ablation may be the choice of treatment in patients with recurrent VT despite appropriate antiarrhythmic treatment and in cases with hemodynamic instability, ECMO support may be a successful strategy to sustain hemodynamic stability.
Ethical approval
The patient’s family gave their informed consent for this case report to be published.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Thiene G, Basso C, Calabrese F, Angelini A, Valente M. Pathology and pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Herz 2000; 25: 210-215. https://doi.org/10.1007/s000590050008
- Corrado D, Perazzolo Marra M, Zorzi A, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020; 319: 106-114. https://doi.org/10.1016/j.ijcard.2020.06.005
- Dalal D, Jain R, Tandri H, et al. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2007; 50: 432-440. https://doi.org/10.1016/j.jacc.2007.03.049
- Goldberger JJ, Arora R, Buckley U, Shivkumar K. Autonomic nervous system dysfunction: JACC focus seminar. J Am Coll Cardiol 2019; 73: 1189-1206. https://doi.org/10.1016/j.jacc.2018.12.064
- Cadrin-Tourigny J, Bosman LP, Nozza A, et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019; 40: 1850-1858. https://doi.org/10.1093/eurheartj/ehz103
- Bosman LP, Sammani A, James CA, et al. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. Heart Rhythm 2018; 15: 1097-1107. https://doi.org/10.1016/j.hrthm.2018.01.031
- Roudijk RW, Verheul L, Bosman LP, et al. Clinical characteristics and follow-up of pediatric-onset arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol 2022; 8: 306-318. https://doi.org/10.1016/j.jacep.2021.09.001
- Ellison KE, Friedman PL, Ganz LI, Stevenson WG. Entrainment mapping and radiofrequency catheter ablation of ventricular tachycardia in right ventricular dysplasia. J Am Coll Cardiol 1998; 32: 724-728. https://doi.org/10.1016/s0735-1097(98)00292-7
- Reithmann C, Hahnefeld A, Remp T, et al. Electroanatomic mapping of endocardial right ventricular activation as a guide for catheter ablation in patients with arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol 2003; 26: 1308-1316. https://doi.org/10.1046/j.1460-9592.2003.t01-1-00188.x
- Trappe HJ, Klein H, Auricchio A, Wenzlaff P, Lichtlen PR. Catheter ablation of ventricular tachycardia: role of the underlying etiology and the site of energy delivery. Pacing Clin Electrophysiol 1992; 15: 411-424. https://doi.org/10.1111/j.1540-8159.1992.tb05137.x
- Pokushalov E, Romanov A, Turov A, Artyomenko S, Shirokova N, Karaskov A. Percutaneous epicardial ablation of ventricular tachycardia after failure of endocardial approach in the pediatric population with arrhythmogenic right ventricular dysplasia. Heart Rhythm 2010; 7: 1406-1410. https://doi.org/10.1016/j.hrthm.2010.06.020
- Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009; 120: 366-375. https://doi.org/10.1161/CIRCULATIONAHA.108.834903
- Thomas V, Lawrence D, Kogon B, Frias P. Epicardial ablation of ventricular tachycardia in a child on venoarterial extracorporeal membrane oxygenation. Pediatr Cardiol 2010; 31: 901-904. https://doi.org/10.1007/s00246-010-9734-5
- Dipchand AI. Current state of pediatric cardiac transplantation. Ann Cardiothorac Surg 2018; 7: 31-55. https://doi.org/10.21037/acs.2018.01.07
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















