Abstract
Background. Giant mucinous cystadenomas are rare in adolescents and young adults.
Case Presentation. We report a mucinous cystadenoma in a 16-year-old postmenarchal girl presented with abdominal distention and pain, and elevated serum CA-125 levels. Radiological evaluations showed a large cystic mass originating from the right ovary. The patient underwent successful surgery with complete resection of the tumor without rupture and the histopathological examination confirmed the diagnosis of a benign mucinous cystadenoma.
Conclusion. The case emphasizes the importance of early diagnosis and the need for total surgical resection without rupture to ensure a favorable outcome in such cases and close follow-up is recommended.
Keywords: mucinous cystadenoma, ovary, adolescent, abdominal mass
Introduction
Ovarian tumors frequently occur in the second decade of childhood and the majority are germ cell tumors.1-5 Epithelial tumors account for 15-20% of pediatric ovarian tumors and they are mostly benign. The most common type of benign epithelial ovarian tumor is cystadenoma, with approximately three-quarters being serous and one-quarter mucinous.3,6,7 Mucinous cystadenomas (MCA) are quite rare in children and adolescents.7-9
Almost all cases of MCA commonly present with complaints of abdominal distention and pain due to a rapidly growing large cystic mass.2,3,6,7,10 The goal of therapeutic surgery is the complete removal of the mass without rupture, preserving normal ovarian tissue whenever possible.8,11 We present here a postmenarchal adolescent patient with a giant mucinous cystadenoma of the ovary that was completely resected.
Case Presentation
A 16-year-old postmenarchal girl was admitted to the pediatric emergency department with one-week history of progressive abdominal distention and periumbilical colicky pain; no history of constipation, diarrhea or vomiting was noted, and she was otherwise in good health. The patient’s most recent menstrual period was 45 days earlier, but she mentioned a small amount of spotting every day. On physical examination, the abdomen was tense and dull to percussion and markedly distended by a huge mass extending from the pelvis to the epigastric area; the bowel sounds were diminished, and no tenderness was noted. Initial laboratory examinations revealed normal complete blood count, serum biochemistry, alpha-feto protein and beta-HCG; serum CA-125 level was elevated at 168.4 U/mL (normal <35 U/mL). An abdominal ultrasound showed a huge multilocular cystic mass extending from the pelvis to the epigastric area which was considered to originate from the right ovary causing dilatation in the right renal collecting system. An abdominal magnetic resonance imaging (MRI) revealed a 28.5×21.1×12.2 cm cystic mass originating from the right ovary with septations; no solid component was detected (Fig. 1).
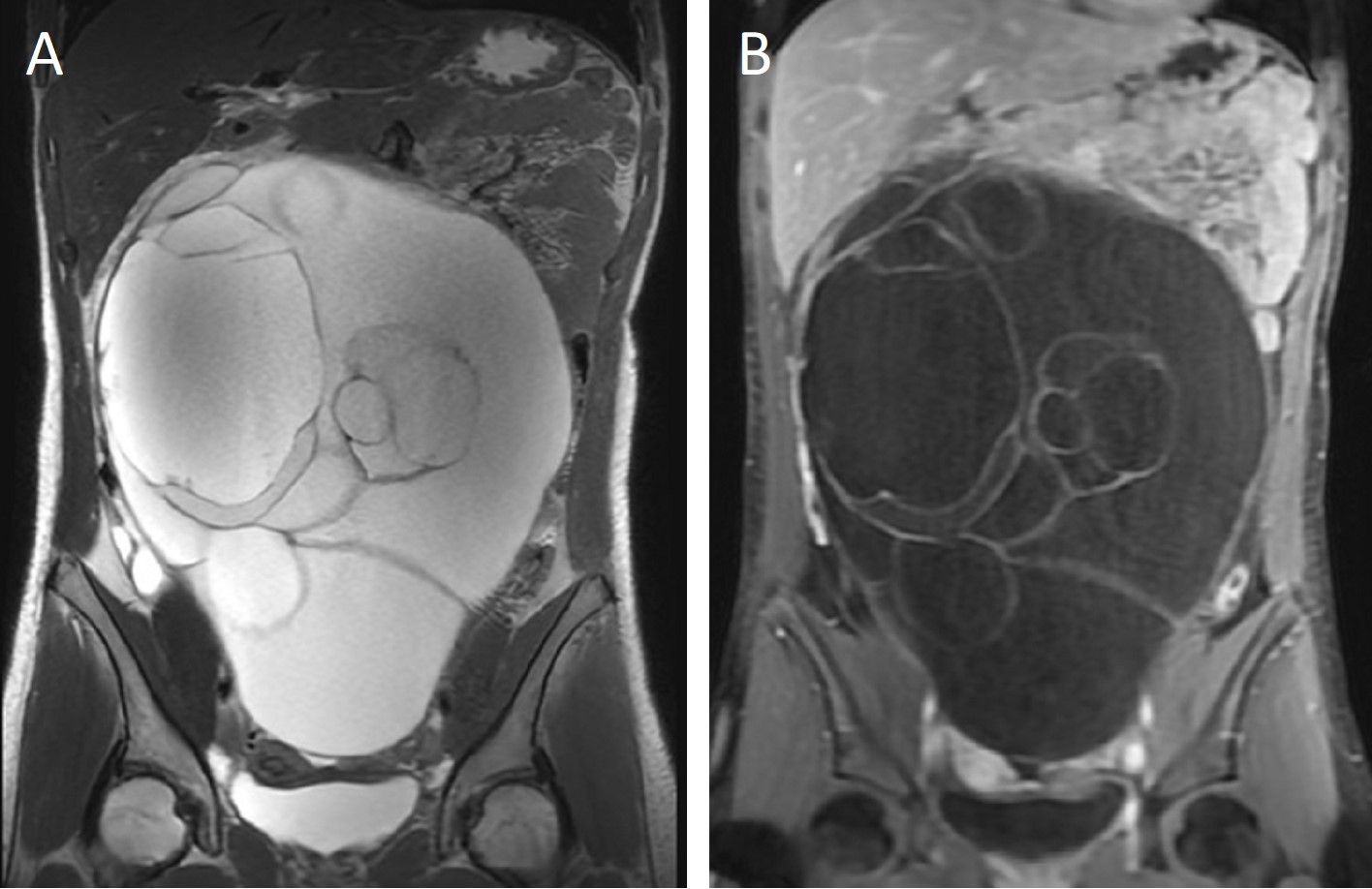
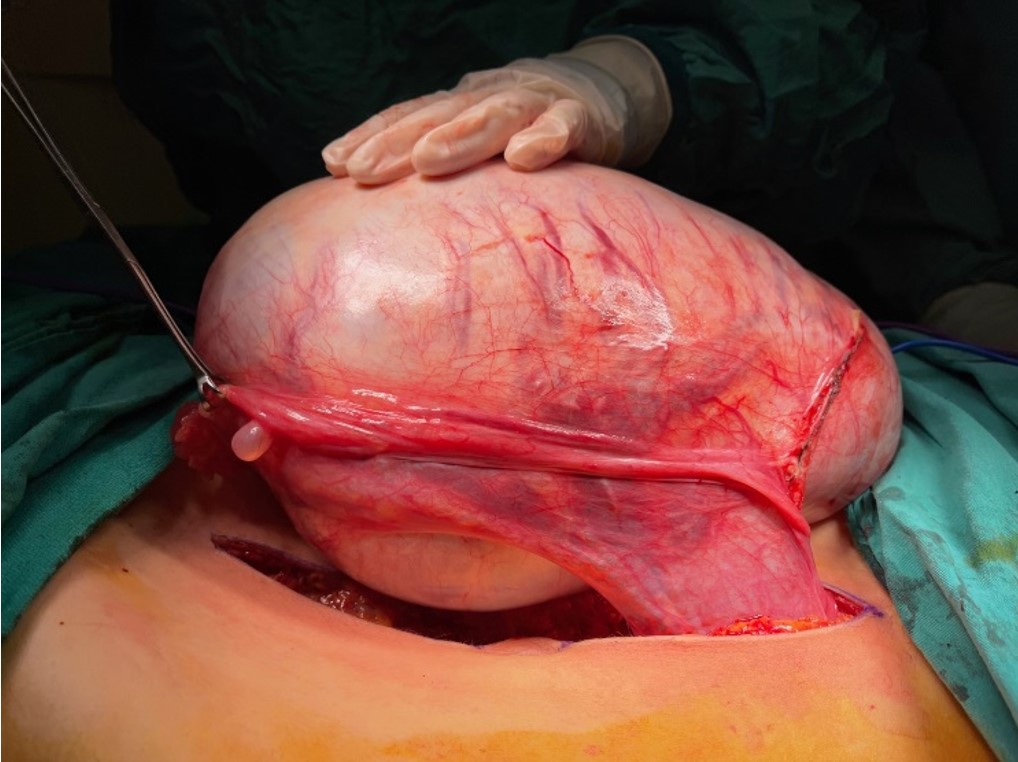
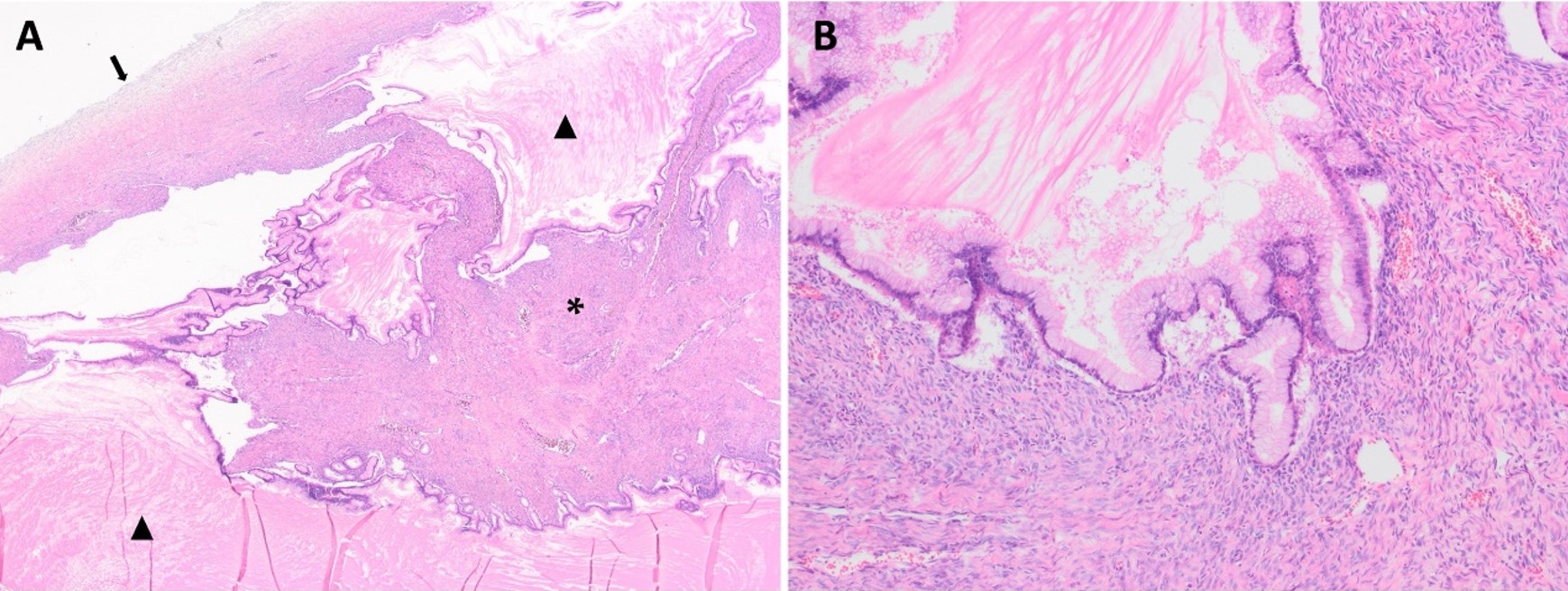
The patient underwent laparotomic oophorectomy, and the huge abdominal mass was totally resected without rupture. Abdominal exploration showed that the left ovary, tuba, and uterus were intact (Fig. 2). The patient had an uneventful postoperative course and was discharged. A gross examination of the resected mass revealed a 27.5×21.5×13 cm cystic mass with an intact capsule and multiple cysts filled with mucinous fluid; solid or papillary components were not detected in the walls of the cysts. The histopathologic examination reported a mucinous cystadenoma with seven reactive sampled lymph nodes (Fig. 3). Cytological examination of the peritoneal fluid showed mesothelial cells, macrophages, and lymphocytes with no tumor cells.
The patient was followed closely, and no further intervention or treatment was needed. The serum CA-125 level was normal in the third postoperative week with a value of 30.3 U/mL. Postoperatively, she had no major complaints and her menstrual periods were regular for the 18 months after surgery. The most recent abdominal ultrasound showed no evidence of residual or recurrent tumor, and a right oophorectomy with normal findings. The patient is under regular follow-up for 20 months after surgery.
Informed consent for the publication was obtained from the patient and her parents.
Discussion
Ovarian tumors are rare in the pediatric population, accounting for only 1% of all pediatric malignancies, with three-quarters being benign.5,12,13 Epithelial neoplasms form a minority of ovarian tumors in childhood, the incidence increases with age, and most occur in the postmenarchal period suggesting that epithelial ovarian tumors are stimulated by hormones.3,4,8,10 Most ovarian epithelial tumors are cystadenomas, mucinous types being less common than the serous and the vast majority are benign.3,6,14-17 In this report, we present a postmenarchal patient with typical clinical, radiological, and pathological characteristics of MCA.
Patients with MCAs commonly present with a variety of clinical symptoms caused by the tumoral mass, such as increased intra-abdominal pressure, distention, pain, vomiting, constipation, pressure on the visceral structures, and sometimes ovarian torsion and rupture.3,5-7,10 Almost all patients have a palpable abdominal mass on examination. Our patient had progressive abdominal distention and pain owing to the rapidly growing tumor in accordance with the literature.
Although most pediatric ovarian masses are benign, early diagnosis is important to prevent complications like ovarian torsion or rupture and to rule out malignant pathologies. Transabdominal ultrasonography is the initial imaging modality to evaluate abdominal masses in the pediatric population. Magnetic resonance imaging or computed tomography are other modalities for further evaluation, differential diagnosis, and management decisions. Radiological assessment of tumor size and complexity may provide valuable insights for stratifying the risk of malignancy in pediatric ovarian tumors prior to histopathological evaluation.10,18
Mucinous cystadenomas appear as a large cystic mass, and the majority are multilocular, characterized by a gelatinous intracystic fluid rich in mucus. The imaging characteristics of MCAs closely reflect their gross pathological features. On cross-sectional imaging, MCA is typically a large unilateral multilocular cystic mass with thin septations and heterogeneous internal components, and also the variable appearance of the cyst fluid owing to differences in the mucin content. Features commonly associated with malignancy include a mass size greater than 10 cm and the presence of solid components, whereas the presence of a simple cyst is highly suggestive of benign pathology.10,15,18
Reports suggest that MCAs are usually unilateral but can grow large, with a mean size of 16-20 cm.12,16 The tumors reported in adolescent patients by Biçer et al.19, Cevik et al.3 and Vizza et al.20 had maximum diameters of 45, 40 and 40 cm, respectively. Watanabe et al.21 reported the largest mucinous cystadenoma case in the pediatric literature with a weight of 11.8 kg. Unfortunately, for our patient the tumor weight was not recorded, but, the largest diameter was measured as 27.5 cm which might be one of the largest tumors reported.
Mucinous ovarian tumors are histopathologically divided into 3 groups: MCA (70%), borderline MCA (10%), and mucinous carcinoma (20%).12,18 MCAs are the most common, usually unilateral, and benign. Microscopically, the tumor consists of cystic spaces lined by tall columnar epithelium with mucinous differentiation.12,18 Consistent with common MCAs, our patient had a mucinous multicystic tumor filled with abundant mucin. Cysts were lined by a single layered epithelium without any complex feature and there was no solid area in the cysts’ walls (Fig. 3).
Serum tumor markers are important in the diagnosis and follow-up of ovarian tumors, elevated levels are usually associated with malignant pathologies.13,22 In our case serum beta-human chorionic gonadotropin (HCG) and α-fetoprotein (AFP) levels were normal, but the CA-125 level was high in the preoperative tests and decreased to normal levels postoperatively. Li et al.10 reported that CA-125 was elevated in 27/58 adolescents with ovarian mucinous tumors. The serum tumor marker CA-125 is specific for epithelial differentiation and plays an important role in the diagnosis and follow-up of epithelial ovarian cancers. The serum CA-125 level can be elevated owing to increased production by the cancer cells which is released from the damaged tumor epithelium at sites of adhesions and epithelial shearing stresses. The levels may also be elevated due to irritation, inflammation, or mechanical stretch of the peritoneal surfaces.23,24 In our patient, the grossly enlarged tumor may have stretched the entire peritoneum, potentially contributing to the elevated serum CA-125 levels. Similar cases have been reported who had very large benign ovarian tumors and elevated serum CA-125 levels.3,19,25 In our patient, despite the elevated serum CA-125 level, histopathological characteristics led to a diagnosis of benign MCA.
Ovarian epithelial tumors in children and adolescents are usually unilateral and benign. Ovarian surgery in children may affect future fertility owing to the removal of the normal ovary or adhesion formation. The current recommended treatment is typically unilateral oophorectomy or salpingo-oophorectomy or cystectomy. If identified and possible, normal ovarian tissue should be preserved.14 The size of the tumor, while not indicative of malignancy, combined with the concern for recurrence often leads the surgeon to perform an oophorectomy.8
During surgery, the large tumor mass should be removed in its entirety without rupture. It is not always possible to accomplish ovary sparing surgery, since MCAs are commonly too big. Cowan et al.8 reported that a significant number of adolescents were treated with a minimally invasive, ovary-sparing approach for large benign MCAs, and this approach was feasible and safe with no evidence of recurrence. In our patient, the tumor was very large, and it was not possible to identify the normal ipsilateral ovary. So, the tumor was removed totally without rupture.
The outcome of MCA is favorable when the tumor is removed without rupture. In the literature, there have been limited cases of recurrent MCA.11,14,25 Intraoperative rupture or spillage of the cyst contents might increase the risk of recurrence. Ben-Ami et al.11 reported recurrence in three patients who experienced intraoperative cyst rupture. During surgery, the condition of the contralateral ovary is also important. If the opposite ovary is grossly normal a biopsy is not indicated. Postoperatively, the patients need to be under follow-up regardless of tumor type due to the possibility of recurrence.11,25 If tumor markers are elevated before surgery, they can be included in the follow-up investigations.
In conclusion, ovarian mucinous cystadenomas are rarely seen in pediatric patients and radiological evaluations play a crucial role in diagnosis, staging, and treatment planning. Total surgical resection without rupture is the mainstay of management. The patients need close follow up after the surgery.
Ethical approval
Informed consent was obtained from the patient and her parents for the publication.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Cemaloğlu M, Kutluk T, Varan A, et al. Primary ovarian tumors in children: a single center experience of 124 patients. Turk J Pediatr 2023; 65: 245-256. https://doi.org/10.24953/turkjped.2022.659
- Morowitz M, Huff D, von Allmen D. Epithelial ovarian tumors in children: a retrospective analysis. J Pediatr Surg 2003; 38: 331-335. https://doi.org/10.1053/jpsu.2003.50103
- Cevik M, Guldur ME. An extra-large ovarian mucinous cystadenoma in a premenarchal girl and a review of the literature. J Pediatr Adolesc Gynecol 2013; 26: 22-26. https://doi.org/10.1016/j.jpag.2012.04.007
- Grapsa D, Kairi-Vassilatou E, Kleanthis C, Dastamani C, Fillipidou A, Kondi-Pafiti A. Epithelial ovarian tumors in adolescents: a retrospective pathologic study and a critical review of the literature. J Pediatr Adolesc Gynecol 2011; 24: 386-388. https://doi.org/10.1016/j.jpag.2011.07.011
- Taskinen S, Urtane A, Fagerholm R, Lohi J, Taskinen M. Metachronous benign ovarian tumors are not uncommon in children. J Pediatr Surg 2014; 49: 543-545. https://doi.org/10.1016/j.jpedsurg.2013.09.019
- Karaman A, Azili MN, Boduroğlu EC, et al. A huge ovarian mucinous cystadenoma in a 14-year-old premenarchal girl: review on ovarian mucinous tumor in premenarchal girls. J Pediatr Adolesc Gynecol 2008; 21: 41-44. https://doi.org/10.1016/j.jpag.2007.09.005
- Sri Paran T, Mortell A, Devaney D, Pinter A, Puri P. Mucinous cystadenoma of the ovary in perimenarchal girls. Pediatr Surg Int 2006; 22: 224-227. https://doi.org/10.1007/s00383-005-1624-1
- Cowan RA, Haber EN, Faucz FR, Stratakis CA, Gomez-Lobo V. Mucinous cystadenoma in children and adolescents. J Pediatr Adolesc Gynecol 2017; 30: 495-498. https://doi.org/10.1016/j.jpag.2017.02.001
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Health Sciences; 2014: 1022-1023.
- Li D, Zhang J, Kiryu S, Zhang X, Wang F. Clinical and CT features of ovarian torsion in infants, children and adolescents. Int J Gynaecol Obstet 2022; 156: 444-449. https://doi.org/10.1002/ijgo.13657
- Ben-Ami I, Smorgick N, Tovbin J, Fuchs N, Halperin R, Pansky M. Does intraoperative spillage of benign ovarian mucinous cystadenoma increase its recurrence rate? Am J Obstet Gynecol 2010; 202: 142.e1-142.e5. https://doi.org/10.1016/j.ajog.2009.10.854
- Brown MF, Hebra A, McGeehin K, Ross AJ. Ovarian masses in children: a review of 91 cases of malignant and benign masses. J Pediatr Surg 1993; 28: 930-933. https://doi.org/10.1016/0022-3468(93)90700-u
- Brookfield KF, Cheung MC, Koniaris LG, Sola JE, Fischer AC. A population-based analysis of 1037 malignant ovarian tumors in the pediatric population. J Surg Res 2009; 156: 45-49. https://doi.org/10.1016/j.jss.2009.03.069
- Knaus ME, Onwuka AJ, Abouelseoud NM, et al. Recurrence rates for pediatric benign ovarian neoplasms. J Pediatr Adolesc Gynecol 2023; 36: 160-166. https://doi.org/10.1016/j.jpag.2022.11.006
- Lack EE, Young RH, Scully RE. Pathology of ovarian neoplasms in childhood and adolescence. Pathol Annu 1992; 27 Pt 2: 281-356.
- Beroukhim G, Ozgediz D, Cohen PJ, et al. Progression of cystadenoma to mucinous borderline ovarian tumor in young females: case series and literature review. J Pediatr Adolesc Gynecol 2022; 35: 359-367. https://doi.org/10.1016/j.jpag.2021.11.003
- Parmentier B, Vaz E, Chabaud-Williamson M, et al. Mucinous cystadenoma arising 3 years after ovarian-sparing surgery for mature teratoma in a child. J Pediatr Surg 2010; 45: E9-E12. https://doi.org/10.1016/j.jpedsurg.2010.05.024
- Marko J, Marko KI, Pachigolla SL, Crothers BA, Mattu R, Wolfman DJ. Mucinous neoplasms of the ovary: radiologic-pathologic correlation. Radiographics 2019; 39: 982-997. https://doi.org/10.1148/rg.2019180221
- Biçer S, Erkul Z, Demiryilmaz I, Peker N. A 9-kg ovarian mucinous cystadenoma in a 14-year-old premenarchal girl. Am J Case Rep 2014; 15: 326-329. https://doi.org/10.12659/AJCR.890862
- Vizza E, Galati GM, Corrado G, Atlante M, Infante C, Sbiroli C. Voluminous mucinous cystadenoma of the ovary in a 13-year-old girl. J Pediatr Adolesc Gynecol 2005; 18: 419-422. https://doi.org/10.1016/j.jpag.2005.09.009
- Watanabe S, Nagashima S, Onagi C, et al. Treatment strategy for pediatric giant mucinous cystadenoma: A case report. Pediatr Rep 2019; 11: 8190. https://doi.org/10.4081/pr.2019.8190
- Taskinen S, Fagerholm R, Lohi J, Taskinen M. Pediatric ovarian neoplastic tumors: incidence, age at presentation, tumor markers and outcome. Acta Obstet Gynecol Scand 2015; 94: 425-429. https://doi.org/10.1111/aogs.12598
- Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer 2021; 1875: 188503. https://doi.org/10.1016/j.bbcan.2021.188503
- Tolman CJ, Vaid T, Schreuder HW. Extremely elevated CA-125 in benign ovarian disease due to stretch of the peritoneum. BMJ Case Rep 2012; 2012: bcr2012006664. https://doi.org/10.1136/bcr-2012-006664
- Fujishima A, Kumazawa Y, Togashi K, Shirasawa H, Sato W, Terada Y. A case of ovarian mucinous cystadenoma in a child that recurred 1 year after surgery. Int J Surg Case Rep 2021; 83: 106006. https://doi.org/10.1016/j.ijscr.2021.106006
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















