Abstract
Background. Gastric teratoma is a rare neoplasm, particularly in neonates, and usually presents as a palpable abdominal mass. However, severe occult gastrointestinal bleeding is uncommon and often misdiagnosed. Imaging studies are crucial for the preoperative diagnosis of neonatal teratoma, but definitive diagnosis relies on pathological examination.
Case Presentation. A 28-day-old boy presented with abdominal distension accompanied by vomiting for 2 days without hematemesis or melena. A complete blood count upon admission showed a hemoglobin level of 37 g/L. Ultrasound and computed tomography scans indicated a large cystic, solid mass in the abdominal cavity (approximately 9.8 × 8.8 × 11.2 cm), containing nodules, septa, calcification, and fat, and causing gastrointestinal compression. The mass was misdiagnosed as lymphangioma with hemorrhage before surgery. During surgery, the upper pole of the tumor was found to be fused with the gastric wall of the greater curvature of the fundus of the stomach, with most of the tumor growing exophytically and a small portion growing into the gastric lumen. The tumor, along with part of the gastric wall at the attachment site, was completely removed. Postoperative pathological examination revealed an immature teratoma grade 1. After discharge, the patient’s growth and development were normal, with no adverse manifestations.
Conclusions. Neonatal gastric teratoma with severe occult gastrointestinal bleeding is extremely rare and hence must be on the list of differential diagnoses of neonatal abdominal mass when a cystic solid mass is found, especially when accompanied by severe anemia without obvious gastrointestinal bleeding. Attention should be paid to the location of the lesion, which is predominantly in the left upper abdomen and has been significantly pushed and displaced by the gastrointestinal tract, and to the imaging characteristics of teratoma such as fat and calcification, which help to exclude other palpable masses encountered during the neonatal period.
Keywords: gastric teratoma, neonatal, immature teratoma, occult gastrointestinal hemorrhage
Introduction
Gastric teratomas are extremely rare, accounting for less than 1% of all teratomas. The first case of gastric teratoma was reported by Eustermann and Sentry in 1922. These rare tumors primarily occur in boys younger than 3 months of age.1 Most gastric teratomas are benign and asymptomatic, often manifesting as an abdominal mass, abdominal distention, vomiting, hematemesis, or melena. Severe occult gastrointestinal bleeding is rare and easily misdiagnosed. Radiological examinations are crucial for the preoperative diagnosis of neonatal teratoma. However, the definitive diagnosis relies on histopathological examination revealing embryonal neuroepithelial tissue accompanying three-germ layer structures. This report details a case of neonatal immature gastric teratoma with severe occult gastrointestinal hemorrhage, initially misdiagnosed as a lymphangioma with hemorrhage. The clinical and imaging characteristics are summarized, causes of misdiagnosis are analyzed, and literature is reviewed to enhance the understanding of this condition among clinicians and radiologists and mitigate future misdiagnosis and mismanagement.
Case Presentation
The patient, a 28-day-old male, was admitted to the hospital due to abdominal distension accompanied by vomiting for 2 days. One day before admission he experienced abdominal distension and vomiting without obvious inducement. The vomiting consisted of stomach contents, and his stool was smooth, yellow, and soft, without blood. On physical examination upon admission, he was alert but exhibited poor mental response, pale face, abdominal distention, a palpable mass in the left upper abdomen with an unclear boundary, and high mobility.
The laboratory examination on the day of admission revealed a white blood cell (WBC) count of 21.3 × 109/L, hemoglobin (Hb) of 37 g/L, procalcitonin (PCT) of 0.77 ng/mL, and C-reactive protein (CRP) of 89.48 mg/L. Color ultrasound showed a large cystic mixed mass in the abdominal cavity (Fig. 1a), measuring 9.8 × 8.8 × 11.2 cm. Multiple bands with uneven thickness, separated echoes, and dotted blood flow signals could be detected along the compartments, and nodular protrusions could be seen in some compartments, causing compressive displacement of the gastrointestinal tract in the abdominal cavity. A computed tomography (CT) scan revealed a large mixed-dense mass in the abdominal cavity, containing calcification, fat, and some solid components (Fig. 1b). Some branches of the superior mesenteric artery were deformed, and the gastrointestinal tract was displaced. The clinical diagnosis was lymphangioma complicated by hemorrhage.
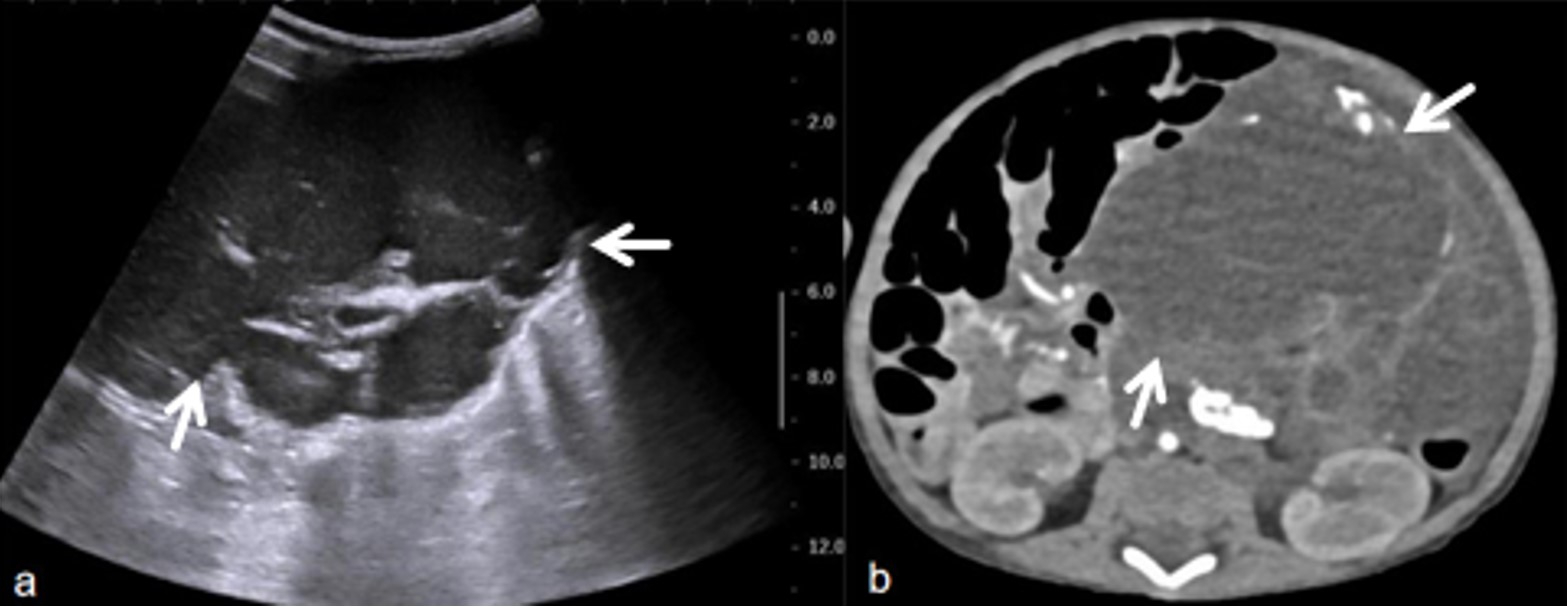
Due to severe anemia, four routine blood tests were performed to evaluate anemia indicators, and 0.25 units of red blood cells were transfused to correct anemia after each test. The huge abdominal mass showed significant extrusion on the surrounding gastrointestinal tract with no suitable needle biopsy path. Concurrently, the persistence of anemia symptoms signified ongoing bleeding within the patient. Therefore, the patient underwent laparoscopic exploratory surgery on day 14 following admission after anemia correction, anti-infection and other symptomatic treatments.
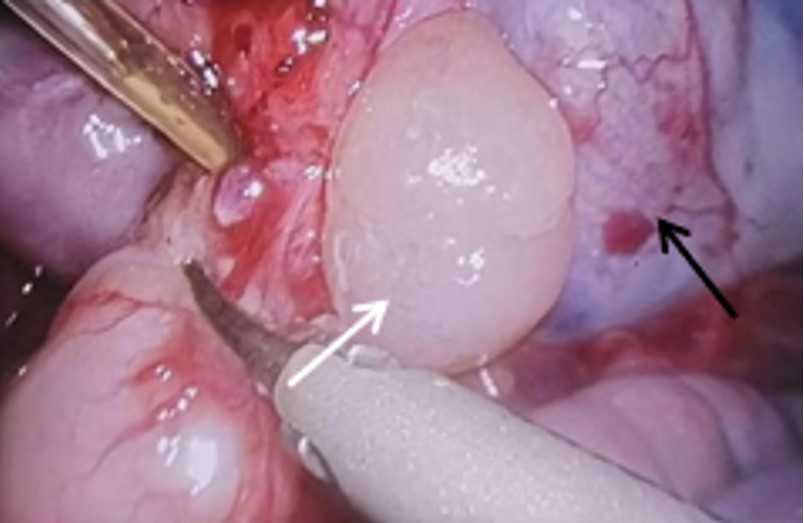
During the operation, the upper pole of the tumor was found to fuse to the gastric wall of the greater curve of the fundus of the stomach (Fig. 2). The gastric wall was cut 1 cm away from the tumor, and most of the tumor was found to be exogenous, with a small part extending into the gastric lumen, measuring approximately 4 × 4 × 3 cm. Dark brown blood clots were observed on the tumor surface and in the gastric lumen. The tumor, along with part of the gastric wall at the attachment site, was completely removed.
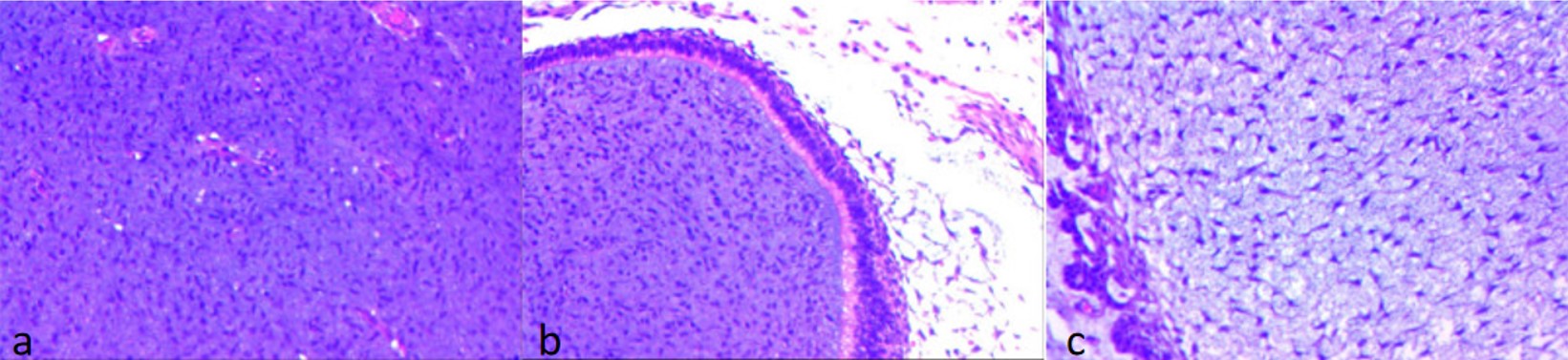
Postoperative pathological biopsy revealed predominantly mature tissues, with some areas containing immature mesenchymal components, leading to a diagnosis of grade 1 immature teratoma (Fig. 3). No tumor tissue was observed on the resection margins. Immunohistochemical staining showed tumor cells positive for CD56 (partial +), S-100 (partial +), and negative for OCT4 and SALL4, with a Ki-67 index of 20%. Postoperatively, the patient received treatments, including fasting water, gastrointestinal decompression, nutritional support, and other treatments. He gradually resumed oral water intake, recovered well, and was discharged. Three months later, follow-up indicated normal growth and development with no adverse manifestations.
Informed consent was obtained from the patient’s family for the publication of this case report.
Discussion
Teratoma is a germ cell tumor formed by the abnormal development of at least two tissues in the outer, middle, and inner layers, occurring both in and outside the gonads. In neonates, teratomas are mostly located outside the gonads, with the sacral tail being the most common site.1 Gastric teratomas are rare in clinical settings, first reported by Eusteman and Sentry in 1922. They mainly occur in boys within 3 months of age and account for <1% of pediatric teratomas.2,3 Over 90% of gastric teratomas occur in the greater curvature of the stomach, with exophytic growth being more common (approximately 60%)1,4 compared to endophytic growth (approximately 30%). Combined exophytic and endophytic growth patterns are rare, and such cases are rarely reported. In this case, the neonatal gastric teratoma occurred in the gastric wall of the greater curvature of the fundus of the stomach. Most teratomas are exogenous, and a few are located in the stomach. Furthermore, most pediatric teratomas are benign and mature, but this patient’s postoperative pathology revealed a grade 1 immature teratoma, which is relatively rare and malignant. The etiology of this condition involves many factors, and its pathogenesis remains unclear.5
The clinical manifestations of gastric teratomas are related to the mass’s size, location, ulceration, and bleeding. Most of them are asymptomatic in the early stages, often presenting later as abdominal mass, abdominal distension, vomiting, hematemesis, and melena. In this case, the gastric teratoma primarily manifested as an abdominal distention accompanied by vomiting, with the vomitus consisting of gastric contents without hematemesis or melena. Upon admission, routine blood examination revealed severe anemia (Hb 37 g/L). During surgery, dark brown blood clots were found on the mass surface and in the gastric lumen, indicating severe occult gastrointestinal bleeding. The probable causes of this bleeding include gastric acid erosion leading to vascular rupture within the tumor, rapid tumor growth causing necrosis and hemorrhage due to insufficient blood supply, or tumor invasion into the gastric mucosa resulting in vascular rupture and hemorrhage. Additionally, the high activity of the abdominal tumor, causing repeated displacement and traction of the gastric wall, may have exacerbated the bleeding, leading to severe anemia in the child. Because gastric bleeding in neonates is relatively hidden, early symptoms are not obvious, resulting in severe hemorrhagic anemia. Emergency surgery should be performed as soon as possible to prevent serious complications.
Immature teratomas contain various amounts of fat, hair, skin, brain tissue, bone, cartilage, and other mature tissues, as well as immature neural and embryonic tissues, such as primitive neural tubes, whose complexity determines the diversity of imaging manifestations.6 Preoperative diagnosis is often based on intratumoral calcification and a mixed cystic-solid mass. Ultrasonography and CT scans can reveal not only heterogeneous masses with varying amounts of cystic and solid components but also fat and calcification, the latter suggesting the diagnosis of teratoma. Differential diagnosis should include other cystic-solid abdominal masses in the child, such as lymphangioma.
The ultrasonographic manifestations of cystic-solid lymphangioma were as follows7: (1) the boundary of the lesion was unclear, and most cases lacked a capsule; (2) irregular shape; (3) uneven internal echo, no echo area of different sizes can be seen, surrounding high or strong echo, the focus is tortuous and expanded tubular structure or honeycomb; (4) color Doppler imaging showed punctate blood flow signal within the lesion. CT findings also have certain characteristics, and the diagnosis of lymphangioma is strongly suggested when the following signs appear8: (1) The shape of the lesion is irregular or bag-like structure, and there are many compartments in the cyst; (2) The volume is huge, but the occupying effect is not apparent; (3) The lesion exhibited “crawling growth” and presents a plastic change with the surrounding tissues. A “vessel crossing sign” can be seen inside the cyst, Indicating poor blood supply. The “creeping growth” of the lesion and the large size with a slight disproportion of the mass effect are the most valuable for diagnosing the disease. The formation of fat signs in lymphangioma may be caused by the growth of surrounding adipose tissue or the accumulation of specific lipid components in lymphatic fluid.
In this report, both CT and ultrasonography indicated a large cystic, solid intraperitoneal mass with a septum, calcification, and a small amount of fat. However, the tumor source could not be determined due to the large mass and insufficient fat in the neonatal abdomen. The child also had severe anemia without hematemesis or melena, which did not conform to the typical characteristics of gastrointestinal bleeding in a neonatal teratoma. This led to a preoperative misdiagnosis of lymphangioma with hemorrhage by clinicians.
Upon reviewing the imaging data, our case showed a regular mass with no “creeping growth” and significant gastrointestinal extrusion, inconsistent with a lymphangioma. At the same time, we found that this cystic-solid mass contained fat and calcification, which was rare in lymphangioma but characteristic in teratoma. Additionally, the tumor was mainly located in the left upper abdomen, suggesting a possible gastric wall origin. Surgery and pathology confirmed that the mass was an immature teratoma originating from the gastric wall.
Currently, the primary treatment for gastric teratoma is complete surgical resection as soon as possible, even if histological examination reveals immature or malignant components and metastasis.9 In this case, the tumor was completely removed via laparoscopy, and the gastric wall was carefully sutured with an enlarged resection margin. Neonatal teratoma generally has a good prognosis, and postoperative transformation is typically not necessary.
However, immature teratomas contain immature components derived from the germ cell layers most commonly neuroectodermal tissue. A few postoperative cases may experience local recurrence, malignant transformation, and even distant metastasis.10,11 The degree of immaturity is correlated with the ultimate prognosis of children. Grade 1 is immature with <10% microscopic foci containing immature elements, grade 2 is immature with 10%–50% of immature elements, and grade 3 is immature with 50% of immature elements. Grade 1 and 2 teratomas may become malignant (grade 3), and malignant teratomas have the potential to metastasize.12 Our case belonged to the grade 1 immature teratoma group. Therefore, the long-term postoperative follow-up is crucial.
In most cases of gastric teratoma, the tumor marker alpha-fetoprotein (AFP) is elevated, which can decrease to normal after surgery. Currently, serum AFP is a monitoring indicator for postoperative tumor remnant and recurrence. Elevated serum AFP levels may also be the only alerting signal for the presence of malignant yolk sac components. However, the diagnostic utility of AFP is low in young infants because of the physiologically elevated levels.12 Therefore, AFP monitoring in this case was not conducted at the first review before surgery and three months after surgery.
In conclusion, neonatal gastric teratoma with severe occult gastrointestinal bleeding is extremely rare and hence, must be on the list of differential diagnoses of neonatal abdominal mass when a cystic solid mass is found, especially when accompanied by severe anemia without obvious gastrointestinal bleeding. Attention should be paid to the location of the lesion, which is predominantly in the left upper abdomen and has been significantly pushed and displaced by the gastrointestinal tract, and to the imaging characteristics of teratoma such as fat and calcification, which help to exclude other palpable masses encountered during the neonatal period.
Acknowledgements
We gratefully thank Dr. Tingting Wu for her contribution to the study deign consultations and comments regarding the manuscript.We also would like to express our sincere gratitude to the doctor Yingxia She for her guidance in the selection of case images. This work was supported by the Natural Science Foundation of Gansu Province (grant No.21JR1RA133).
Ethical approval
Informed consent was obtained from the patient’s family for the publication of this case report.
Source of funding
This study was supported by Natural Science Foundation of Gansu Province (No.21JR1RA133).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Yoon SE, Goo HW, Jun S, Lee IC, Yoon CH. Immature gastric teratoma in an infant: a case report. Korean J Radiol 2000; 1: 226-228. https://doi.org/10.3348/kjr.2000.1.4.226
- Caballes AB, Dungca LBP, Uy MEV, Torralba MGC, Embuscado CMG. Hydrops fetalis and neonatal abdominal compartment syndrome continuum from immature gastric teratoma: a case report. BMC Pediatr 2020; 20: 186. https://doi.org/10.1186/s12887-020-02090-0
- Lu B, Yang L. Gastric teratoma invasion and bulb fistula formation in an adult: report of one case and literature review. J Int Med Res 2019; 47: 5849-5854. https://doi.org/10.1177/0300060519869722
- Dutta R, Agarwala S, Madhusudhan KS, Das P. Immature gastric teratoma: a rara avis. Indian J Pathol Microbiol 2022; 65: 203-205. https://doi.org/10.4103/ijpm.ijpm_564_21
- Attard TM, Omar U, Glynn EF, Stoecklein N, St Peter SD, Thomson MA. Gastric cancer in the pediatric population, a multicenter cross-sectional analysis of presentation and coexisting comorbidities. J Cancer Res Clin Oncol 2023; 149: 1261-1272. https://doi.org/10.1007/s00432-022-03972-9
- Shinkai T, Masumoto K, Chiba F, et al. Pediatric ovarian immature teratoma: histological grading and clinical characteristics. J Pediatr Surg 2020; 55: 707-710. https://doi.org/10.1016/j.jpedsurg.2019.04.037
- Jianhang W, Bin C, Qiuyue C. High frequency ultrasonographic signs and diagnostic value of lymphangioma in children. Chinese and Foreign Medical Research 2021; 19: 56-59. https://doi.org/10.14033/j.cnki.cfmr.2021.26.017
- Liping G, Chenguang G, Wenfei L, Shaohui M, Ming Z, Chen N. CT and clinical characteristics of rare abdominal lymphangioma in adults. Modern Oncology 2016; 24: 1812-1816. https://doi.org/10.3969/j.issn.1672-4992.2016.11.039
- Selvarajan N, Kathirvelu G, Ramalingam TR, Mokrala UBS, Karunakaran P, Tharanendran H. Immature gastric teratoma: a case report. J Indian Assoc Pediatr Surg 2021; 26: 464-465. https://doi.org/10.4103/jiaps.JIAPS_36_21
- Ukiyama E, Endo M, Yoshida F, et al. Recurrent yolk sac tumor following resection of a neonatal immature gastric teratoma. Pediatr Surg Int 2005; 21: 585-588. https://doi.org/10.1007/s00383-005-1404-y
- Gilcrease MZ, Brandt ML, Hawkins EP. Yolk sac tumor identified at autopsy after surgical excision of immature sacrococcygeal teratoma. J Pediatr Surg 1995; 30: 875-877. https://doi.org/10.1016/0022-3468(95)90770-x
- Aihole JS, Babu MN, Jadhav V, Javaregowda D. Gastric teratoma: an unusual presentation and location. Indian J Med Paediatr Oncol 2017; 38: 563-565. https://doi.org/10.4103/ijmpo.ijmpo_182_16
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















