Abstract
Background. In children admitted to the pediatric intensive care unit (PICU), early detection of risk factors and alarming indicators improves the prognosis and may even save lives. Several prognostic markers and scores have been studied in children who are seriously ill. Recently, surface triggering receptor expressed on myeloid cells-1 (sTREM1) has been studied in many infectious and non-infectious settings; however, there is little information on critically ill children. Our aim is to evaluate the sTREM1 level in critically ill children and assess its prognostic role.
Method. A prospective observational study was conducted in a tertiary care hospital. 70 critically ill children and 50 healthy controls were enrolled in the study. Demographic, clinical, and laboratory data were obtained. sTREM1 level was assessed on admission to the PICU. Patients with conditions affecting immunity were excluded. The primary outcome was to assess the level of sTREM1 in both patients and controls. Secondary outcomes were mortality, morbidities as sepsis, need for mechanical ventilation, and PICU stay.
Results. The level of sTREM1 was significantly higher in patients than in controls (850 pg/mL, interquartile range [IQR] 510.0- 1375.0 vs. 67.5 pg/mL, IQR 40.0- 85.0; p<0.001). sTREM1 level was significantly higher in non-survivors (p <0.001), patients with sepsis (p = 0.0028), and in patients who were mechanically ventilated (p <0.001). sTREM1 level had a significant positive correlation with the duration of PICU stay (r=0.624, p <0.001), and the duration of mechanical ventilation (r=0.527, p <0.001). On ROC curve analysis, sTREM1 was the most significant diagnostic marker compared to lactate and procalcitonin, at a cutoff value of 680 pg/mL, with a sensitivity of 93.8% and a specificity of 61.1% with an area under the curve of 0.862.
Conclusion. In critically ill children, sTREM1 has prognostic and diagnostic values. There were associations between sTREM1 and the severity of the disease. To validate our results, subgroup analysis and multicenter trials are necessary.
Keywords: sTREM1, critically ill children, sepsis, markers, triggering receptors
Introduction
Critically ill children need special care and monitoring, and are usually cared for in the pediatric intensive care unit (PICU). PICU populations are usually heterogeneous, as there will be several medical and surgical disorders, each differing in their hemodynamic, inflammatory state, and presence or absence of infections.1 Early identification of risk factors and alarming signs in the PICU population helps to save their lives and improve the prognosis. Also, risk stratification of the patients helps to intervene early and allocate the appropriate level of care for each patient according to their condition, thus improving the patient outcome and saving resources, especially in resource-limited countries.1-3
Numerous prognostic indicators and scores have been examined in critically ill children. Mortality scores have been the most reliable of these indicators, namely Pediatric RISk of Mortality (PRISM) and Paediatric Index of Mortality-2 (PIM2) scores.2,3 The search for prognostic markers has gained popularity in pediatric research.1,4 Procalcitonin (PCT) and C-reactive protein (CRP), two frequently used diagnostic markers, are less sensitive and specific. As a result, research is being conducted on novel markers of acute inflammation, among them are surface triggering receptor expressed on myeloid cells-1 (sTREM1) and interleukin-6 (IL-6).4
Triggering receptor expressed on myeloid cells-1 (TREM1) is an immunoglobulin superfamily member that is a cell surface protein. The human TREM1 is a type of DAP12-related receptor that is a 30-kDa glycoprotein located on the 6p21 chromosome.5-7 Mature monocytes, neutrophils, and macrophages all express it. As a pro-inflammatory marker, TREM1 increases inflammation upon exposure to extracellular bacterial and fungal pathogens.8 TREM1 has been investigated as a marker for pneumonia, neonatal sepsis, neonatal ventilator-associated pneumonia (VAP)9, pediatric multisystem inflammatory syndrome (MIS-C)10, pediatric sepsis, and septic shock11, and in oncology patients with febrile neutropenia.12 A multitude of research has found that it has a prognostic value in these populations. Additionally, sTREM1 has been investigated in non-infectious situations recently, with varying degrees of efficacy and limited data on pediatric patients. However, sTREM1 has not been investigated in many other critical illnesses in children. Hence, the aim of this study is to assess the role of sTREM1 as a prognostic marker in critically ill children.
Materials and Methods
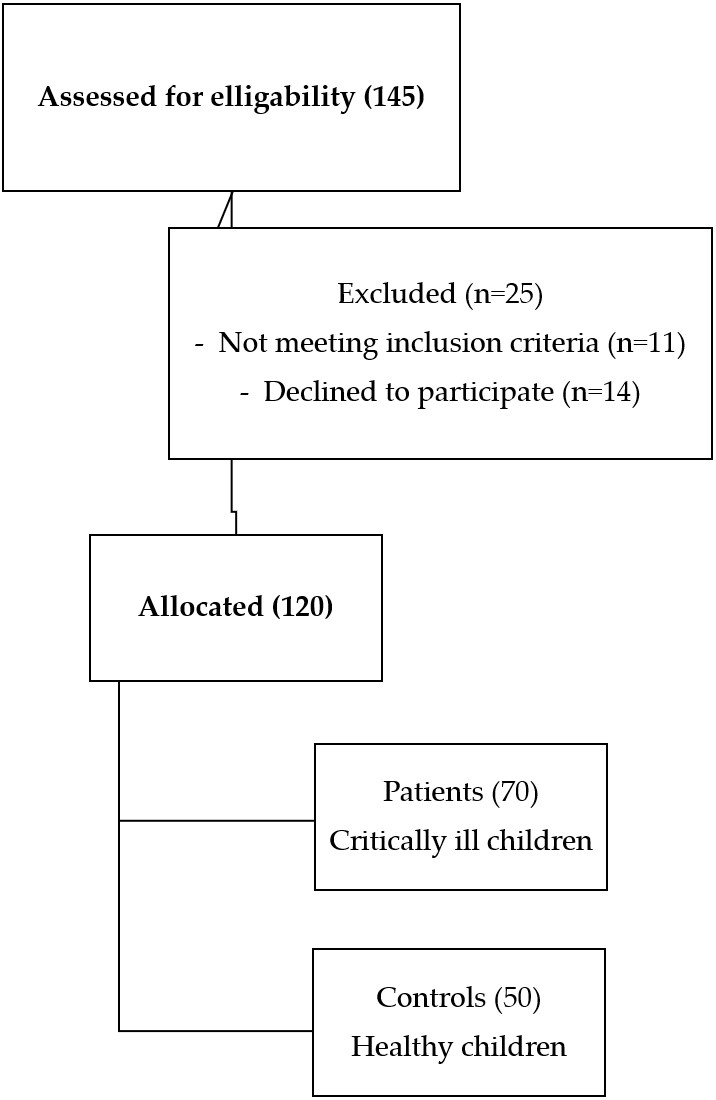
This was a prospective observational study that was carried out on 70 critically ill children who were admitted to the PICU of Menoufia University Hospital in the period from May 2022 to August 2023 (Fig. 1). Additionally, 50 healthy age- and sex-matched children were enrolled as a control group. A written informed consent was obtained from the parents of the participant children after explaining the aim and procedures of the study. The study was approved by the Ethics Committee of Menoufia University – Faculty of Medicine (IRB 5-20222ped27) Ethics Committee.
All critically ill children admitted to the PICU with infectious and non-infectious conditions, aged 1 month to 18 years were included in this study. Patients with congenital and acquired immunodeficiency, malignancy, acute and chronic liver diseases, and chronic inflammatory diseases, and those receiving immunosuppressive medications were excluded.
Detailed history, physical and clinical examination for all participants were recorded. Oxygen saturation (SpO2), severity of the underlying medical condition, PRISM score, diagnosis category according to primary diagnosis and comorbidities were assessed.
The primary outcome was the level of sTREM1 in patients and controls. Secondary outcomes were the occurrence of mortality, sepsis, need for mechanical ventilation, and length of PICU stay.
Laboratory investigations such as complete blood count, kidney function tests (serum urea and creatinine), liver function tests, serum electrolytes, arterial blood gas, serum lactate, CRP, PCT and sTREM1 levels were conducted.
Serum samples were collected for sTREM1 measurement for all patients on admission to the PICU. For serum separation, two milliliters of blood were drawn from a peripheral vein and placed in special clot activator tubes. The blood was then aliquoted and frozen at -80°C right away for additional analysis. The Human TREM-1 (DuoSet ELISA kit, R and D Systems, Minneapolis, MN, USA) was used to quantify sTREM1 by the manufacturer’s instructions. The findings were expressed as pg/mL.
Statistical Package for the Social Sciences (SPSS) version 23 (Armonk, NY: IBM Corp.) was used on a personal computer to gather, tabulate, and statistically analyze the data. There were two sections of statistics, descriptive statistics (the presentation of quantitative data as median and range when data were non-normally distributed) and analytical statistics (chi-square test and Student t test). Mann-Whitney test is a nonparametric test (U) used when data are non-normally distributed. The ROC (receiver operating characteristic) curves were utilized for comparing the diagnostic role of different markers. Logistic regression test was performed for prediction of mortality by sTREM1 and other variables. The P value of < 0.05 was considered a significant level.
Results
The patient group consisted of 70 critically ill children, and the control group consisted of 50 healthy children. The main demographic, clinical, and laboratory characteristics are demonstrated (Table I).
| Numerical data presented as median (interquartile range), categorical data as n (%). BMI: Body mass index, MV: Mechanical ventilation, NA: Not applicable, SGOT: Serum glutamate oxaloacetate transaminase, SGPT: Serum glutamate pyruvate transaminase, sTREM1: Surface triggering receptor expressed on myeloid cells-1, *: p<0.05. | |||
| Table I. Demographic, clinical, and laboratory data of the patients and controls. | |||
| Variable | Patients (N = 70) | Controls (N = 50) | P- value |
| Age (months)an (IQR) | 13 (2.0- 192.0) | 18 (4.0- 48.0) | 0.270 |
| Male sex | 38 (54.2%) | 19 (38%) | 0.17 |
| Weight (kg) | 9 (3.0- 53.0) | 11.3 (6.0- 17.0) | 0.078 |
| Height (cm) | 72.5 (49.0- 162.0) | 84 (63.0- 105.0) | 0.023* |
| BMI (kg/m2) | 5.9 (2.7-16.8) | 6.9 (4.7- 14.5) | 0.115 |
| Primary diagnosis: | |||
| Respiratory disease | 32 (45.71%) | NA | NA |
| Cardiac disease | 18 (25.71%) | ||
| Neurologic | 11 (15.71%) | ||
| Others | 9 (12.86%) | ||
| Hemoglobin (g/dL) | 10.2 (2.80- 15.90) | 11.9 (10.5- 12.5) | <0.001* |
| Platelet count (x103/µL) | 322 (16.0- 797.0) | 345 (228.0- 453.0) | 0.745 |
| White blood cells (x103/µL) | 12.8 (4.90-41.00) | 9.9 (8.4-12.5) | <0.001* |
| Urea (mg/dL) | 35 (11.0-120.0) | 24 (16.0-32.0) | <0.001* |
| Creatinine (mg/dL) | 0.7 (0.20-5.70) | 0.5 (0.2-0.7) | 0.118 |
| SGOT (U/L) | 45 (10.0-454.0) | 26 (16.0-65.0) | 0.003* |
| SGPT (U/L) | 32 (10.0-539.0) | 18 (14.0-25.0) | <0.001* |
| Na (mEq/L) | 139 (126.0-156.0) | 139 (136.0-145.0) | 0.853 |
| K (mEq/L) | 4.2 (1.90-7.20) | 4.3 (3.4-4.8) | 0.981 |
| Ionized Ca (mmol/L) | 0.9 (0.70-9.40) | 1.1 (0.90-1.20) | 0.005* |
| sTREM1 (pg/mL) | 850 (510.0- 1375.0) | 67.5 (40.0- 85.0) | <0.001* |
| Mortality | 16 (22.9%) | NA | NA |
| Need for MV | 29 (41.4%) | NA | NA |
| Sepsis | 25 (35.7%) | NA | NA |
The sTREM1 level was significantly higher in the patient group compared to controls (Table I). sTREM1 level was associated with different outcomes; it was significantly higher in patients who were ventilated than those non-ventilated, and in those who died than those who survived. Also, it was significantly higher in patients who developed sepsis than in those that did not (Table II).
| Mann-Whitney U test. sTREM1 levels presented as median (interquartile range) in pg/mL. PICU: Pediatric Intensive Care Unit, sTREM1: Surface triggering receptor expressed on myeloid cells-1, *: Significant. | ||
| Table II. Association between sTREM1 levels and different outcomes. | ||
| sTREM1 | P value | |
| Survivor | 760 (510-1300) | <0.001* |
| Non-survivor | 1130 (660-1375) | |
| Sepsis | 950 (625-1375) | 0.0028* |
| No sepsis | 800 (510-1375) | |
| Mechanical ventilation | 1010 (510- 1375) | < 0.001* |
| No mechanical ventilation | 740 (550- 1125) | |
There was a statistically significant positive correlation between sTREM1 with PICU stay (r=0.624, p=0.001) and with duration of mechanical ventilation (rs=0.527, p<0.0001).
On comparing surviving and non-surviving patients regarding different parameters and outcomes, we noticed a significant difference in PRISM score, sTREM1 level, lactate, PCT, CRP, serum transaminases, and need for mechanical ventilation (Table III).
| Numerical data presented as median (interquartile range) and analyzed with Mann-Whitney U test, categorical data presented as n (%) and analyzed with chi-square test. BMI: Body mass index, CRP: C-reactive protein, MV: Mechanical ventilation, SGOT: Serum glutamic-oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase, TREM: Triggering receptor expressed on myeloid cells, *: Significant. | |||
| Table III. Comparison between survived and non-survived children regarding demographic, clinical, and laboratory parameters. | |||
| Survived (n=54) Median (IQR) |
Non- survived (n=16) Median (IQR) |
P value | |
| Age (months) | 12 (2-192) | 13.5 (2-160) | 0.900 |
| Weight (kg) | 8.8 (3-53) | 9.5 (3.5-39) | 0.905 |
| Height (cm) | 72.5 (49-162) | 73 (53-152) | 0.801 |
| BMI (kg/m2) | 5.9 (2.7-16.8) | 6.3 (3.1-12.8) | 0.978 |
| Male sex | 27 (50.00%) | 11 (68.75%) | 0.186 |
| PRISM score | 3 (0-8) | 7 (3-9) | <0.001* |
| Procalcitonin (ng/mL) | 0.7 (0.2-4.5) | 1.5 (0.5-18.9) | <0.001* |
| Lactate (mmol/L) | 2.3 (0.5-4.0) | 3.5 (1.30-3.80) | <0.001* |
| sTREM1 (pg/mL) | 790 (510-1300) | 1130 (660-1375) | <0.001* |
| Mechanical ventilation | 13 (24.07%) | 16 (100.00%) | <0.001* |
| Hemoglobin (g/dL) | 10.2 (6.5-15.9) | 10.2 (2.8-12.1) | 0.675 |
| Platelet count (x103/µL) | 313.5 (16.0-797.0) | 367.0 (45.0-672.0) | 0.454 |
| White blood cells (x103/ µL) | 12.6 (5.5-30.1) | 13.0 (4.9-41.0) | 0.944 |
| CRP (mg/dL) | 24.0 (0.0-158.0) | 42.5 (12.0-180.0) | 0.296 |
| Urea (mg/dL) | 35.0 (11.0-120.0) | 35.0 (15.0-69.0) | 0.752 |
| Creatinine (mg/dL) | 0.6 (0.2-5.7) | 0.7 (0.2-2.1) | 0.927 |
| SGOT (U/L) | 40.0 (10.0-352.0) | 72.0 (27.0-454.0) | 0.005* |
| SGPT (U/L) | 25.0 (10.0-539.0) | 45.5 (18.0-357.0) | 0.007* |
| Na (mEq/L) | 139.0 (126.0-156.0) | 140.0 (126.0-150.0) | 0.580 |
| K (mEq/L) | 4.3 (2.2-7.2) | 4.0 (1.9-5.2) | 0.125 |
| Ca (mmol/L) | 0.9 (0.7-9.4) | 1.1 (0.7-4.2) | 0.566 |
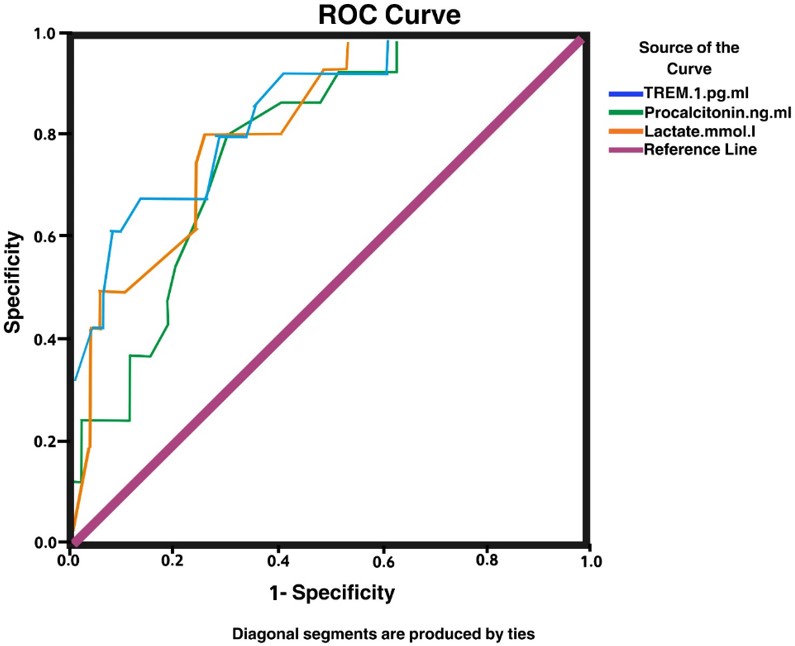
On ROC curve analysis, the area under the curve for sTREM1, PCT, and lactate were 0.862, 0.793, and 0.810, respectively. Additionally, sTREM1 had a higher area under the curve compared to PCT and lactate levels, and it was the most significant diagnostic tool marker in critically ill children at a cutoff value of 680.0 pg/mL, with a sensitivity of 93.8%, and a specificity of 61.1% with an area under the curve of 0.862 (Table IV, Fig. 2).
| AUC: Area under the ROC Curve, CI: Confidence interval, sTREM1: Surface triggering receptor expressed on myeloid cells-1. | ||||||||
| Table IV. ROC curve to detect sTREM1, procalcitonin and lactate levels as a marker in critically ill children. | ||||||||
| Variable(s) | AUC | Std Error | P value | Sensitivity | Specificity | Cutoff value | 95% CI of AUC | |
| Lower | Upper | |||||||
| sTREM1 (pg/mL) | 0.862 | 0.051 | <0.001 | 93.8% | 61.1% | 680.0 | 0.76 | 0.96 |
| Procalcitonin (ng/mL) | 0.793 | 0.057 | <0.001 | 68.80 | 35.69 | 1.25 | 0.682 | 0.905 |
| Lactate (mmol/L) | 0.810 | 0.059 | <0.001 | 80.13 | 40.70 | 14.50 | 0.713 | 0.930 |
Binary logistic regression analysis for the parameters affecting survival and mortality rate showed that PRISM score, CRP, lactate, PCT, and sTREM1 were negatively associated with survival (Table V).
| BMI: Body mass index, CI: Confidence interval, CRP: C-reactive protein, PRISM: Pediatric Risk of Mortality Score, SGOT: serum glutamate oxaloacetate transaminase, SGPT: Serum glutamate pyruvate transaminase, sTREM1: Surface triggering receptor expressed on myeloid cells-1, *significant. | |||||||
| Table V. Binary logistic regression analysis for the for the parameters affecting survivor and mortality rate. | |||||||
| Variables | B | S.E. | Wald | Sig. | Exp(B) | 95% C.I. for EXP(B) | |
| Lower | Upper | ||||||
| Age (months) | -0.005 | 0.007 | 0.573 | 0.449 | 0.995 | 0.981 | 1.008 |
| Weight (kg) | -0.024 | 0.030 | 0.636 | 0.425 | 0.976 | 0.919 | 1.036 |
| Height (cm) | -0.013 | 0.012 | 1.152 | 0.283 | 0.987 | 0.964 | 1.011 |
| BMI (kg/m2) | -0.074 | 0.098 | 0.576 | 0.448 | 0.928 | 0.766 | 1.125 |
| PRISM score | 0.720 | 0.171 | 17.779 | 0.0001 | 2.053 | 1.470 | 2.869 |
| Hemoglobin (g/dL) | -0.273 | 0.145 | 3.547 | 0.060 | 0.761 | 0.573 | 1.011 |
| Platelet count (x103/µL) | 0.002 | 0.002 | 0.754 | 0.385 | 1.002 | 0.998 | 1.005 |
| White blood cells (x103/µL) | 0.038 | 0.039 | 0.950 | 0.330 | 1.039 | 0.962 | 1.123 |
| CRP (mg/dL) | 0.014 | 0.007 | 4.063 | 0.044 | 1.014 | 1.000 | 1.027 |
| Urea (mg/dL) | -0.002 | 0.013 | 0.033 | 0.855 | 0.998 | 0.972 | 1.024 |
| Creatinine (mg/dL) | -0.149 | 0.372 | 0.160 | 0.689 | 0.861 | 0.415 | 1.787 |
| SGOT (U/L) | 0.008 | 0.004 | 3.299 | 0.069 | 1.008 | 0.999 | 1.017 |
| SGPT (U/L) | 0.001 | 0.004 | 0.082 | 0.774 | 1.001 | 0.993 | 1.010 |
| Na (mEq/L) | -0.061 | 0.058 | 1.089 | 0.297 | 0.941 | 0.840 | 1.055 |
| K (mEq/L) | -0.606 | 0.407 | 2.220 | 0.136 | 0.545 | 0.246 | 1.211 |
| Ca (mmol/L) | -0.004 | 0.330 | 0.000 | 0.990 | 0.996 | 0.522 | 1.900 |
| Procalcitonin (ng/mL) | -0.970 | 0.323 | 9.000 | 0.003 | 2.637 | 1.400 | 4.969 |
| Lactate (mmol/L) | -0.403 | 0.054 | 3.613 | 0.050 | 1.109 | 0.997 | 1.233 |
| sTREM1 (pg/mL) | -0.005 | 0.002 | 6.868 | 0.009 | 1.005 | 1.001 | 1.009 |
Discussion
Much research on critically ill children has focused on identifying predictors of mortality and other important outcomes. The quick identification of such people emphasizes how crucial it is to make correct and speedy identification upon admission to the PICU so that proper therapies can be started. This offers the potential to lead to better outcomes.1-4
In this study, we investigated the role of sTREM1 in critically ill children. This marker has been investigated in many infectious and inflammatory conditions in children and adults. However, the data on critically ill children is sparse. We found that the sTREM1 level was significantly higher in patients than in controls, and it was also significantly higher in non-survivors than in survivors, which emphasizes its prognostic role. This could be explained by conditions with severe inflammation express much proinflammatory mediators and metalloproteinases that cleaves the soluble form sTREM1 from the membrane anchored TREM1. This sTREM1 can amplify the innate immune response. Neutrophil degranulation, cell survival, and strong production of tumor necrosis factor (TNF)-α, IL-6, IL-1β, IL-8, monocyte chemoattractant protein-1 (MCP-1), IL-12p40, and granulocyte monocyte colony stimulating factor (GM-CSF) by monocytes/macrophages and dendritic cells are all outcomes of activation of the TREM-1 pathways. These processes increase inflammation during infection by various pathogens, including influenza, dengue, and hepatitis C, as well as in chronic inflammatory diseases.
Similar results were illustrated in a study by Şen et al.8, who showed higher sTREM1 levels in patients with sepsis and septic shock, and this also had a prognostic role with regards to mortality and other outcomes. They explained their findings by the predominant pro-inflammatory mediators in patients with more severe forms of sepsis and septic shock, highlighting the prognostic value of sTREM1 in the early detection of patients requiring special care.
Additionally, Yang et al.9 demonstrated that sTREM has diagnostic and prognostic roles in children with VAP. Conversely, sTREM1 was also investigated in children with febrile neutropenia; however, other markers, such as IL-8, were found to be superior in predicting the prognosis.9 Gonçalves et al.10 investigated the role of sTREM1 in children with MIS-C, and found that it has value as an early screening tool in children with MIS-C.
In relation to different outcomes, the current study investigated the level of sTREM1 in patients who developed sepsis and those who did not. We found that patients with sepsis had significantly higher sTREM1 levels. This indicates that sTREM1 is not only a marker of inflammation but also a marker of infection. This may be explained by the fact that sTREM1 levels are higher in sepsis due to the host organism’s cytokine storm, which promotes inflammation that worsens with the severity of sepsis.10
Similar results in pediatric sepsis research were found by Leligdowicz et al.13, who showed that sTREM1 can be used as a rapid triage test in febrile African children at risk of sepsis. Şen et al.8 investigated the role of sTREM1 and IL-8 in differentiating sepsis, severe sepsis, and septic shock in children, and found that patients with septic shock had higher sTREM1 levels than those with severe sepsis. However, in sepsis, there was no correlation between sTREM1 levels and death, and they recommended that when determining the prognosis of child sepsis, sTREM1 readings should be applied with caution. Conversely, Smok et al.4 found that PCT and IL-6 had a greater role in systemic inflammatory response syndrome, sepsis detection and prognosis, and further studies are required regarding the role of sTREM1.
Adult studies on patients with COVID-19 have investigated the role of sTREM1 as a prognostic marker and have stated that it is possible to measure sTREM1 in bodily fluids. Severe cases of inflammatory illnesses generally lead to a rise in this biomarker, which is considered an inflammatory and predictive measure of sepsis. Severe and critical types of COVID-19 are the primary causes of the rise in sTREM1 plasma levels, indicating that this molecule may be a useful predictor of a poor prognosis.10
In relation to mechanical ventilation, the current study demonstrated a higher sTREM1 level in patients who were ventilated than those who did not require mechanical ventilation, which indicates that sTREM1 could be used as a marker of poor outcomes. There was a significant positive correlation between sTREM1 and the duration of mechanical ventilation. Similar to our results, much research found that sTREM1 was higher in patients who were ventilated, especially those who had VAP, both in children and neonates.9
In relation to PICU length of stay, there was a significant positive correlation between sTREM1 level and duration of PICU stay. This may be related to many factors; those children had more severe disease, more need for mechanical ventilation, and developed sepsis. These results may be utilized to predict critically ill children’s prognosis in advance and to guide early intervention to stop their rapid deterioration.
In relation to other biomarkers of infection and inflammation, several markers of inflammation and infection have been used in PICU to predict the severity and prognosis of critically ill children, of these are CRP, albumin, lactate, and PCT besides clinical scores as PRISM. Elevated blood lactate or impaired lactate clearance have been considered prognostic markers in different PICU patients including shock, sepsis, inflammatory conditions, clinically suspected sepsis.1,14-16 and postoperative cardiac patients with congenital heart disease.17 CRP has been used as a marker correlating with infection and inflammation. PCT was presented as a novel and innovative indicator of infection. Bacterial endotoxins and exotoxins, together with inflammatory cytokines, are known to induce PCT.12 Assicot et al.18 have reported a high serum PCT in patients with sepsis and bacterial infection.
In this study, ROC curve analysis showed that sTREM1 was the most significant diagnostic tool marker in critically ill children compared with lactate and PCT, at a cutoff value of 680 pg/mL, with a sensitivity of 93.8% and a specificity of 61.1% with an area under the curve (AUC) of 0.862, as compared to PCT and lactate levels. However, binary logistic regression analysis indicates that PRISM score, PCT, and lactate in comparison with sTREM1 are statistically significant predictors of the outcome of critically ill children, where every one-unit change in sTREM1 level will increase mortality by 1.005 while the change in PRISM score, PCT, lactate, and CRP are more influential in influencing the outcome in comparison with sTREM1 (odds ratios 2.053, 2.617, 1.109 and 1.014, respectively).
Similar to our study, much research has compared different markers in many conditions in the PICU. Arslan et al.1 have reported that a lactate cut-off value of 5.55 mmol/L resulted in an AUC of 0.79 in a study performed on 1109 critically ill children.15 Another study conducted on patients with septic shock showed that lactate levels higher than 4 mmol/L have a good prognostic role in mortality.16
Conversely, Miedema et al.12 investigated the role of multiple markers in diagnosing severe bacterial infection in oncology children with febrile neutropenia, and found that sTREM1 and other markers such as CRP and PCT are less useful markers for early detection compared to IL-8.
Interestingly, combining biomarkers has shown to have a greater value in diagnosis and determining the prognosis of critically ill children; Sdougka et al.19 mentioned that studies conducted on patients with VAP have shown significant discriminative value of the combined biomarkers in differentiating infectious and non-infectious causes.20-22 Additionally, measuring a panel of biomarkers at different times in addition to clinical variables and scores has a better predictive power in those populations. Abdelaziz et al.17 have shown that combining markers as lactate and central venous oxygen saturation (ScvO2) have a greater prognostic role in children after surgery for congenital heart disease.
We compared survivors with non-survivors in terms of mortality and discovered that, along with other parameters including CRP, PCT, serum glutamate pyruvate transaminase, and PRISM score, non-survivors had considerably greater sTREM1. In order to investigate the factors influencing mortality, we also performed a logistic regression analysis. The results showed that PRISM score, CRP, lactate, PCT, and sTREM1 were all adversely correlated with survival.
Based on the results of our work and previous research, sTREM1 may be considered an accurate triage tool because it is triggered by cytokines and inflammatory mediators and its level increases as the severity of the primary sickness increases. As so, it could facilitate early decision-making and intervention.
Strong points in this study are the precise inclusion criteria, comprehensive data collecting on clinical outcomes and standard-of-care testing. It is remarkable that these biomarkers can predict a patient’s likelihood of survival when they have hyperinflammatory conditions like sepsis. The prompt identification of these patients highlights how important it is to accurately and promptly identify them upon PICU admission in order to initiate the appropriate medication. There is a chance that this will produce better results.
The study also has several limitations: Subgroup analysis is required for better interpretation. The study’s single-center design limits the findings’ applicability to other populations. A multicenter study design is recommended for better validation of the results. Additionally, sTREM1 is better measured serially than in a single measurement; this might correlate better with the disease progression.
Conclusion
sTREM1 has a diagnostic and prognostic value in critically ill children. Compared to other markers of infection and inflammation, sTREM1 has shown comparable correlation with disease severity. Multicenter studies are required to validate our results with subgroup analysis.
Acknowledgements
The authors express their gratitude to all patients participated in the research and their caregivers.
Ethical approval
The study received approval from the Ethical Committee of Faculty of Medicine – Menoufia University (date: 01-05-2022, IRB number: 5-2022ped27).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Arslan G, Besci T, Özdemir G, et al. Predictive value of PRISM-4, PIM-3, CRP, Albumin, CRP/Albumin Ratio and Lactate in critically ill children. Children (Basel) 2023; 10: 1731. https://doi.org/10.3390/children10111731
- Slater A, Shann F; ANZICS Paediatric Study Group. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med 2004; 5: 447-454. https://doi.org/10.1097/01.PCC.0000138557.31831.65
- Yousef RAM, El Gendy FM, Abd El Aziz AA. Prognostic scoring systems in pediatric ICUs: pediatric risk of mortality III versus pediatric index of mortality 2. Alexandria Journal of Pediatrics 2019; 32: 27-32. https://doi.org/10.4103/AJOP.AJOP_12_19
- Smok B, Domagalski K, Pawłowska M. Diagnostic and prognostic value of IL-6 and sTREM-1 in SIRS and Sepsis in children. Mediators Inflamm 2020; 2020: 8201585. https://doi.org/10.1155/2020/8201585
- Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis 2003; 187(Suppl 2): S397-S401. https://doi.org/10.1086/374754
- Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology 2008; 213: 701-713. https://doi.org/10.1016/j.imbio.2008.07.008
- Qian L, Weng XW, Chen W, Sun CH, Wu J. TREM-1 as a potential therapeutic target in neonatal sepsis. Int J Clin Exp Med 2014; 7: 1650-1658.
- Şen S, Kamit F, İşgüder R, et al. Surface TREM-1 as a prognostic biomarker in pediatric sepsis. Indian J Pediatr 2021; 88: 134-140. https://doi.org/10.1007/s12098-020-03355-3
- Yang ZQ, Mai JY, Zhu ML, et al. Soluble triggering receptors expressed on myeloid cells-1 as a neonatal ventilator-associated pneumonia biomarker. Int J Gen Med 2021; 14: 4529-4534. https://doi.org/10.2147/IJGM.S315987
- Gonçalves GS, Correa-Silva S, Zheng Y, et al. Circulating sTREM-1 as a predictive biomarker of pediatric multisystemic inflammatory syndrome (MIS-C). Cytokine 2023; 161: 156084. https://doi.org/10.1016/j.cyto.2022.156084
- Duramaz BB, Ankay N, Yesilbas O, et al. Role of soluble triggering receptor expressed in myeloid cells-1 in distinguishing SIRS, sepsis, and septic shock in the pediatric intensive care unit. Arch Pediatr 2021; 28: 567-572. https://doi.org/10.1016/j.arcped.2021.06.001
- Miedema KG, de Bont ES, Elferink RF, et al. The diagnostic value of CRP, IL-8, PCT, and sTREM-1 in the detection of bacterial infections in pediatric oncology patients with febrile neutropenia. Support Care Cancer 2011; 19: 1593-1600. https://doi.org/10.1007/s00520-010-0987-6
- Leligdowicz A, Conroy AL, Hawkes M, et al. Risk-stratification of febrile African children at risk of sepsis using sTREM-1 as basis for a rapid triage test. Nat Commun 2021; 12: 6832. https://doi.org/10.1038/s41467-021-27215-6
- Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 2009; 9: 30-33. https://doi.org/10.7861/clinmedicine.9-1-30
- Scott HF, Brou L, Deakyne SJ, Kempe A, Fairclough DL, Bajaj L. Association between early lactate levels and 30-day mortality in clinically suspected sepsis in children. JAMA Pediatr 2017; 171: 249-255. https://doi.org/10.1001/jamapediatrics.2016.3681
- Jat KR, Jhamb U, Gupta VK. Serum lactate levels as the predictor of outcome in pediatric septic shock. Indian J Crit Care Med 2011; 15: 102-107. https://doi.org/10.4103/0972-5229.83017
- Abdelaziz AA, ElGendy FM, Hegazy AA, et al. Prognostic value of combined central venous oxygen saturation and lactate in pediatric patients after cardiac surgery. Egyptian Pediatric Association Gazette. 2023; 71: 84. https://doi.org/10.1186/s43054-023-00230-6
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341: 515-518. https://doi.org/10.1016/0140-6736(93)90277-n
- Sdougka M, Simitsopoulou M, Volakli E, et al. Evaluation of five host inflammatory biomarkers in early diagnosis of ventilator-associated pneumonia in critically ill children: a prospective single center cohort study. Antibiotics (Basel) 2023; 12: 921. https://doi.org/10.3390/antibiotics12050921
- Refaat A, Affara N, Abdel-fatah W, Hussein T, El-gerbi M. Diagnostic accuracy of inflammatory biomarkers in bronchoalveolar lavage from patients with ventilator-associated pneumonia. Egypt J Chest Dis Tuberc 2014; 63: 723-730. https://doi.org/10.1016/j.ejcdt.2014.03.003
- Fagon JY. Biological markers and diagnosis of ventilator-associated pneumonia. Crit Care 2011; 15: 130. https://doi.org/10.1186/cc10050
- Salluh JIF, Souza-Dantas VC, Póvoa P. The current status of biomarkers for the diagnosis of nosocomial pneumonias. Curr Opin Crit Care 2017; 23: 391-397. https://doi.org/10.1097/MCC.0000000000000442
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.