Graphical Abstract
Abstract
Background. Immune thrombocytopenia (ITP) is a multifactorial disease involving environmental and genetic factors. This study aimed to evaluate the association of a single nucleotide polymorphism (SNP) rs3853839 in the Toll-like receptor 7 (TLR7) gene with susceptibility to ITP and its clinical features.
Methods. This retrospective, observational, case-control study was conducted on 172 pediatric patients with ITP and 170 healthy children. Genomic DNA was extracted from peripheral blood and genotyped via a snapshot technique.
Results. The serum TLR7 mRNA in the case group (1.129±0.536) was significantly higher than that in the control group (0.851 ± 0.298) (p<0.001). Female patients with the GG genotype and male patients with the G/(-) genotype demonstrated the highest level of TLR7 mRNA (1.478±0.522 and 1.280±0.590, respectively) (p<0.0001), whereas female patients with the CC genotype and male patients with the C/(-) genotype showed the lowest level of TLR7 mRNA (0.752±0.171 and 0.732±0.218, respectively) (p<0.0001). The severity and chronic progression of ITP was significantly increased in female patients with the GG genotype and male patients with the G/(-) genotype (p<0.05). However, TLR7 rs3853839 polymorphism was not significantly associated with corticosteroid sensitivity and disease recurrence (p>0.05).
Conclusions. This study suggests that TLR7 rs3853839 may be a key genetic factor in the susceptibility and severity of ITP disease, providing new insights into disease progression and severity prediction. These findings present significant insights into the pathogenesis of ITP and may serve as a foundation for developing personalized treatment strategies tailored for pediatric patients with ITP.
Keywords: immune thrombocytopenia (ITP), TLR7, genetic polymorphism, genotype
Introduction
Primary immune thrombocytopenia (ITP) is a highly complex autoimmune disease.1 Its etiology is still not fully understood. Genetic and environmental factors play essential roles in the pathogenesis of ITP.2 The clinical features of ITP can include increased antibody-mediated platelet destruction or antibody-mediated inhibition of platelet production, leading to insufficient platelet production.3 The International Working Group (IWG) defines ITP as newly diagnosed (diagnosis to 3 months), persistent (3 to 12 months from diagnosis), or chronic (lasting >12 months).4
Toll-like receptors (TLRs) play essential roles in responses against microbial agents, inflammatory pathways, and the regulation of innate immune responses.5 Disturbances in the innate immune response can precipitate the onset and progression of ITP.6 TLRs induce the development and differentiation of T-cell subsets, including Th1, Th2, Th17, and Tregs. They also modulate the development and functions of Tregs through mediated signals and can impact the development of atopic disorders. Dysregulated TLR expression or genetic variations can contribute to imbalances in Th1 or Th2 immunity levels.7-9 In patients with ITP, there is an observed imbalance in CD4+ T cell subsets, characterized by a skewed Th1/Th2 balance that favors Th1 and a skewed Th17/Treg balance that favors Th17. Consequently, there is an elevated production of Th1 and Th17 cytokines.10,11
Toll-like receptor 7 (TLR7) gene is found in X chromosome Xp22.2, which encodes the TLR7 protein. TLR7 serves as an intracellular pattern recognition receptor and is associated with multiple polymorphisms that are potentially associated with human disease.12 In the investigation into the pathogenesis of ITP, whole-blood gene expression profiling from ITP patients was conducted, revealing the involvement of TLR7 in the pathogenesis of ITP.13
Although studies have shown that TLR7 may play an important role in the pathogenesis of ITP, the specific genetic variation and its functional effects still need further exploration. Previous studies on ITP gene polymorphism mainly focused on the PTPN22 gene14, HDAC3 gene15, and FOXP3 gene16, while the study of TLR7 gene polymorphism and ITP has not been reported.
TLR7 expression is associated with TLR7 rs3853839 C/G polymorphism, which has been shown to impact TLR7 mRNA turnover in genetic studies.17 The rs3853839 C/G SNP, which is located in the 3′ untranslated region of the TLR7 gene, has been associated with increases in TLR7 mRNA and protein expression.18 Numerous studies have indicated a correlation between the rs3853839 C/G SNP and various autoimmune diseases, including systemic lupus erythematosus (SLE)12, knee osteoarthritis19, and autoimmune thyroid disease.20 To our knowledge, to date no studies have been undertaken to clarify the relationship between TLR7 rs3853839 C/G polymorphism and ITP. Therefore, it is essential to investigate the rs3853839 C/G single nucleotide polymorphism to enhance our understanding of the pathogenesis and clinical characteristics associated with ITP.
The main objective of this study was to evaluate the association of the TLR7 rs3853839 C/G SNP with susceptibility to ITP and its clinical features.
Materials and Methods
Study design
This retrospective, observational, case-control study was conducted on 172 pediatric patients with ITP, and 170 healthy children were included as a control group. The study was conducted from January 2020 to January 2023. All the patients agreed to participate in the study and signed a written informed consent form before enrollment. The diagnosis of ITP is based on the updated international consensus report.21 In this study, the clinical information of the children was collected, and all the children were followed for at least 1 year. Patients with a follow-up duration of less than one year or incomplete records were excluded from the study.
These definitions were developed in accordance with ITP Working Group guidelines to study the clinical characteristics and follow-up treatment outcomes of ITP.21
Study definitions
- Primary ITP: isolated thrombocytopenia (peripheral blood platelet count <100×109 /L) in the absence of other conditions associated with thrombocytopenia.
- Newly diagnosed ITP: within 3 months from diagnosis.
- Persistent ITP: between 3 and 12 months from diagnosis.
- Chronic ITP: lasting for >12 months.
- Severe ITP: presenting with bleeding sufficient to start treatment or occurrence of new bleeding symptoms necessitating additional treatment mediation with an alternative agent or an increased dose.
Response to treatment
All children with ITP were administered high-dose dexamethasone through intravenous infusion. The treatment was given at 0.6 mg/kg daily, with a maximum dose allowed as 40 mg over four days. The response after steroid treatment was evaluated as follows:
Corticosteroid sensitivity: after standard glucocorticoid treatment, the platelet count significantly increased and stabilized at a safe level (≥ 30×109 /L), effectively managing bleeding symptoms.
Corticosteroid resistance: platelet counts below 30×109 /L, less than a twofold increase of baseline platelet count, or the presence of bleeding symptoms.
Recurrence: decrease in platelet counts below 30×109 /L or less than twice the baseline or the presence of bleeding symptoms following treatment response.
Sample collection
The sterile venipuncture was used to extract 3-5 mL of venous blood from each patient into an EDTA anticoagulant tube. A blood DNA extraction kit (Qiagen, Maryland, USA) was used to store the samples in the same vacuum tank at -20 °C until DNA extraction.
DNA amplification and isolation
Whole-blood DNA was extracted via the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). DNA concentration and purity were determined using a spectrophotometer NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA, USA), and all DNA samples were diluted to working concentrations of 50 ng/µL.
Amplification PCR and snapshot assay
The SNaPshot assay is a mini-sequencing method widely used to detect polymorphisms in individual genes or the whole genome. The assay is sensitive and straightforward, and the results can be automatically evaluated using software.22 Genotyping of the TLR7 rs3853839 gene locus was performed via the Snapshot technique.
The total volume of each PCR mixture was 25 μL: 5 μL of DNA, 12.5 μL of prepared Taq Red PCR master mix (Bioline), 1 μL of each primer, and 5.5 μL of nuclease-free water. The forward primer sequence of TLR7 rs3853839 was as follows: AACCAATTGCTTCCGTGTCA. The reverse primer sequence of TLR7 rs3853839 was as follows: GTTGCTGTATCAAGTGTGCAGA. The PCR cycle conditions were as follows: 1 cycle of 95 °C predenaturation for 30 seconds followed by 40 cycles of 95 °C denaturation for 10 seconds and 60 °C annealing and extension for 30 seconds. The PCR products of each SNP were examined via 2.0% agarose gel electrophoresis and digested with the corresponding restriction enzymes (Thermo Scientific, USA) according to the manufacturer’s protocol. The digested products were electrophoresed on a 3% agarose gel containing ethidium bromide and then visualized via UV transmission.
Statistical analysis
The Helmholtz Centre website (Germany) was used to calculate the Hardy-Weinberg equilibrium (HWE) of TLR7 rs3853839 genotypes. SPSS 23 software (IBM Corp, NY, USA) was used for statistical analysis of the current data. Normally distributed measurement data are presented as the means ± SDs. The chi-square test was used to compare categorical variables and assess the distribution of TLR7 SNPs in the association between TLR7 rs3853839 and ITP susceptibility, corticosteroid sensitivity, disease severity, disease recurrence, and disease progression. The risk associated with individual genotypes or alleles was calculated as the odds ratio (OR) with their 95% confidence intervals (95% CI). Differences between various groups were compared via a one-way analysis of variance and p<0.05 indicated a statistically significant difference.
Results
Essential characteristics of the research subjects
This study included 172 pediatric patients with ITP and 170 children in the control group. Cases and controls were matched in age and gender. The mean age of disease onset among cases was 6.065±3.283 years, while in the control group, it was 5.939±3.344 years (p=0.688) (Table I). The male-to-female ratio in the case group was 1.17:1, while in the control group, it was 1.24:1 (p=0.725) (Table I). The serum TLR7 mRNA in the case group (1.129±0.536) was significantly higher than that in the control group (0.851±0.298) (p<0.001) (Table I and Fig. 1). Female patients with the GG genotype and male patients with the G/(-) genotype demonstrated the highest level of TLR7 mRNA (1.478±0.522 and 1.280±0.590, respectively) (p<0.0001). Whereas female patients with the CC genotype and male patients with the C/(-) genotype showed the lowest level of TLR7 mRNA (0.752±0.171 and 0.732±0.218, respectively) (p<0.0001) (Table I and Fig. 1). The remaining clinical and laboratory data of the case group encompassed platelet count, initial bleeding event, corticosteroid sensitivity, disease severity at the time of sampling, disease recurrence, and disease progression, which are shown in Table I.
| ITP, immune thrombocytopenia; N/A, not applicable; TLR7, Toll-like receptor 7. | |||
| Table I. Clinical and demographic characteristics of participants. | |||
| Characteristics |
|
|
|
| Age of disease onset (range) (years) |
|
|
|
| Sex (male: female) |
|
|
|
| Relative expression of TLR7 mRNA |
|
|
|
| TLR7 mRNA expression by genotype | |||
| Female | |||
| GG |
|
|
|
| CG |
|
|
|
| CC |
|
|
|
| Male | |||
| G/(-) |
|
|
|
| C/(-) |
|
|
|
| Platelet count (×109 /L) |
|
|
|
| ≤30×109 /L (n, %) |
|
|
|
| >30×109 /L (n, %) |
|
|
|
| Initial bleeding event (n, %) |
|
||
| Purpura |
|
|
|
| Ecchymosis |
|
|
|
| Wet purpura |
|
|
|
| Corticosteroid sensitivity (n, %) |
|
||
| Complete response |
|
|
|
| Response |
|
|
|
| No response |
|
|
|
| Disease severity at sampling (n, %) |
|
||
| Severe |
|
|
|
| Non-severe |
|
|
|
| Disease recurrence (n, %) |
|
||
| Recurrent |
|
|
|
| Non-recurrent |
|
|
|
| Disease progression (n, %) |
|
||
| Chronic ITP |
|
|
|
| Non-chronic ITP |
|
|
|
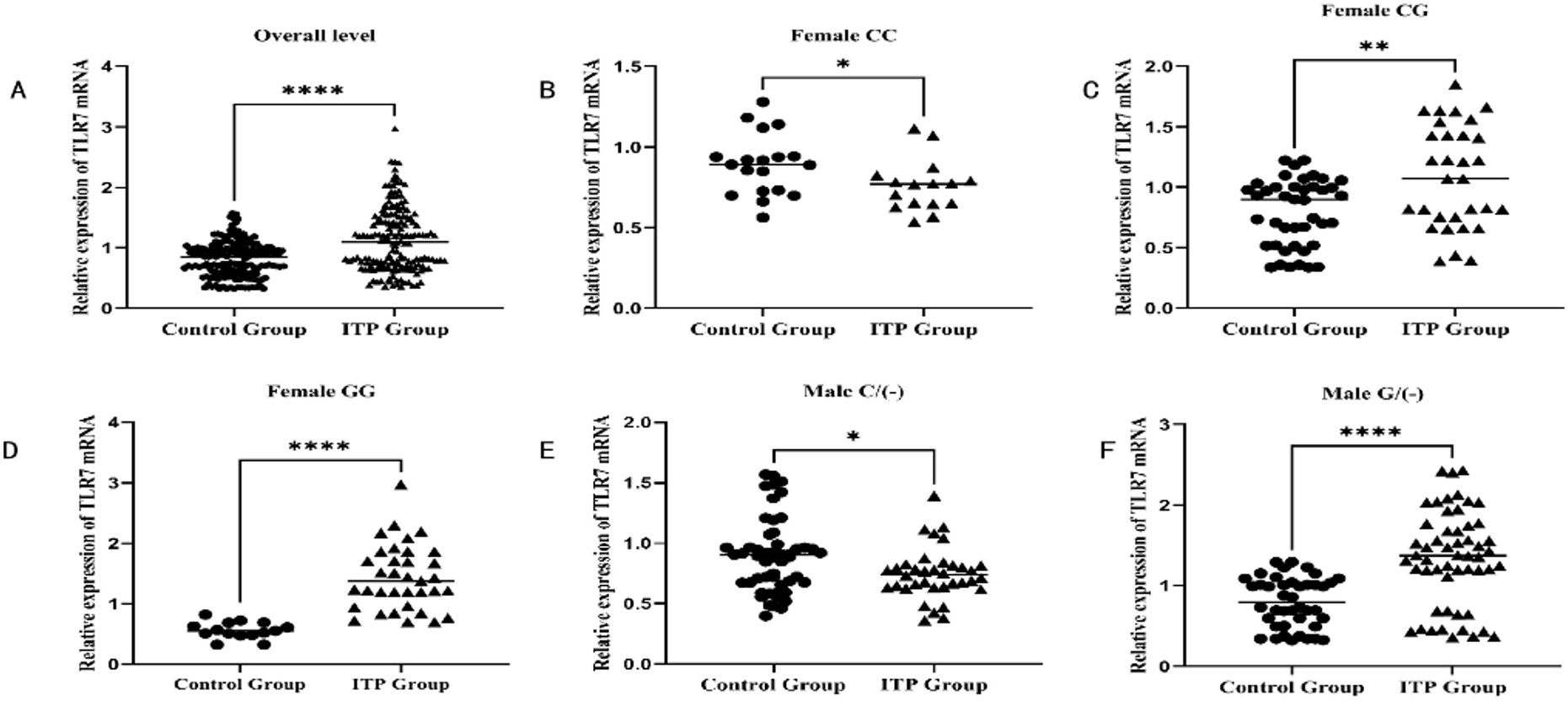
ITP, immune thrombocytopenia; TLR7, toll-like receptor 7.
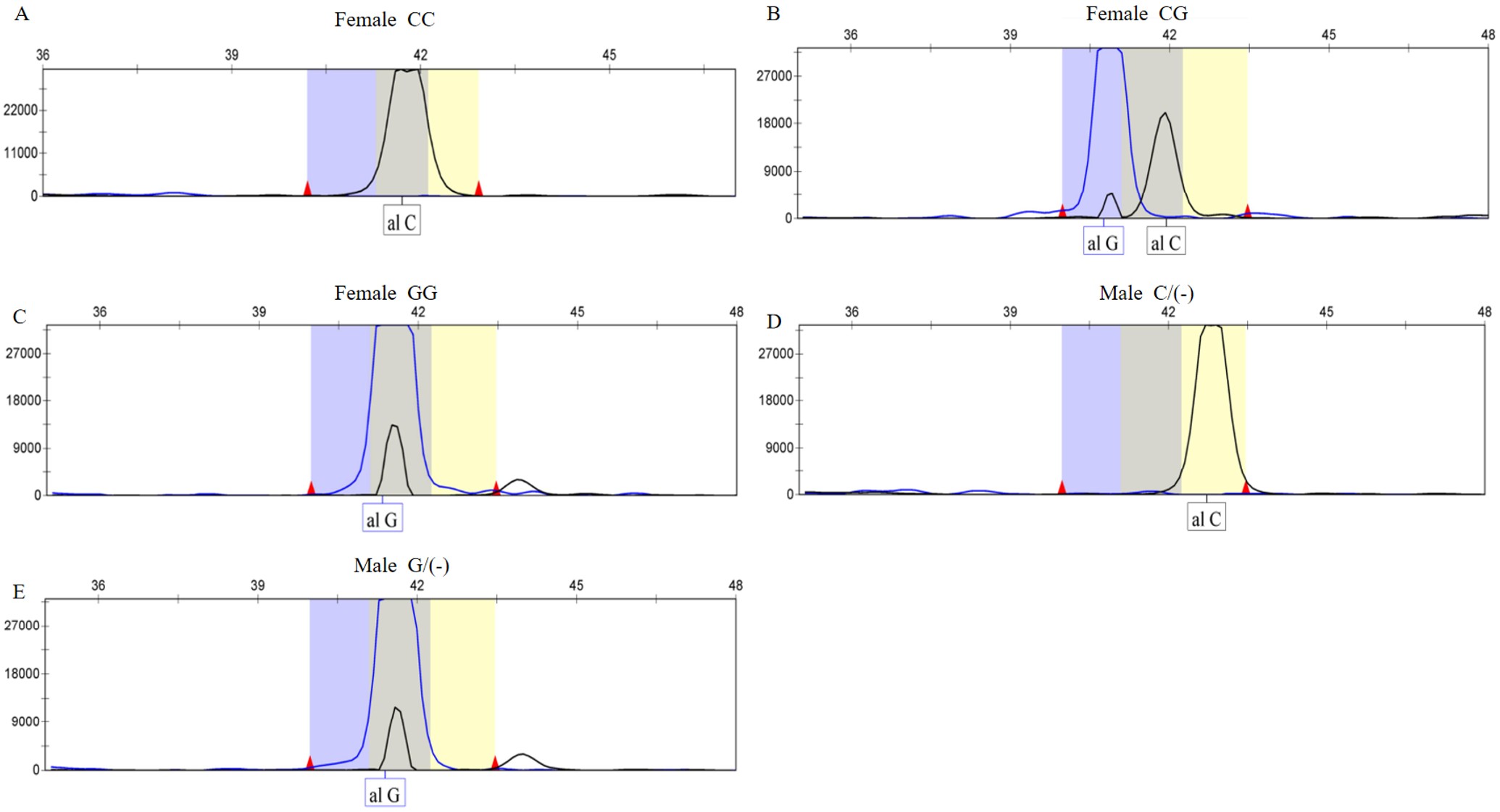
TLR7 rs3853839 PCR amplification products and SNaPshot sequencing results
SNaPshot sequencing results indicate that blue peaks correspond to the G base, and black peaks correspond to the C base. Both blue and black peaks signify the CG base. As the TLR7 gene is situated on the X chromosome, female children exhibited the GG, CC, and CG genotypes (Fig. 2, A, B, and C), while male children showed the G/(-) and C/(-) genotypes (Fig. 2, D and E).
TLR7 rs3853839 C/G genotype, allele, and gene frequency distributions
The TLR7 rs3853839 GG genotype frequency in female patients was significantly higher compared to the control group (χ2=6.505, p=0.039) (Table II). The TLR7 rs3853839 G/(-) genotype frequency in male patients was significantly higher compared to the control group (χ2=3.968, p=0.046; OR=1.857, 95% CI: 1.034-3.336) (Table II). The TLR7 rs3853839 G allele gene frequency in ITP patients was significantly higher compared to the control group (χ2=6.146, p=0.013; OR=1.567, 95% CI: 1.098-2.238) (Table II). The TLR7 rs3853839 GG carrying gene frequency in ITP patients was significantly higher compared to the control group (χ2=5.826, p=0.016; OR=2.337, 95% CI: 1.164-4.693) (Table II).
The relationship between TLR7 rs3853839 C/G gene polymorphism and corticosteroid sensitivity in pediatric patients with ITP
All cases were classified into corticosteroid-sensitive and corticosteroid-resistant cohorts based on their reactivity to corticosteroid therapy (n=107 and 65, respectively). The distribution of genotypes between the two groups was compared; however, no significant correlation was observed between the TLR7 rs3853839 genotypes and corticosteroid sensitivity in pediatric patients with ITP (p>0.05, Table III). This indicates that the TLR7 rs3853839 polymorphism is not associated with ITP in relation to the response to corticosteroid therapy.
The relationship between TLR7 rs3853839 C/G gene polymorphism and the severity of pediatric patients with ITP
All cases were classified into the severe ITP group and non-severe ITP group (n=70 and 102, respectively). Among female ITP patients with different genotypes of TLR7 rs3853839, the proportion of GG genotype in severe ITP patients was 19 (55.88%), which was significantly higher than that in non-severe ITP patients 14 (29.17%), and this difference was statistically significant (χ2=6.371, p=0.041, Table III). Among male ITP patients with different genotypes of TLR7 rs3853839, the proportion of G/(-) genotype in severe ITP patients was 28 (77.78%), which was significantly higher than that in non-severe ITP patients 31 (57.41%), and this difference was again statistically significant (χ2=3.969, p=0.046, Table III). This indicates a potential correlation between the TLR7 rs3853839 polymorphism and the severity of ITP disease.
The relationship between TLR7 rs3853839 C/G gene polymorphism and disease recurrence
All cases were classified into the recurrent ITP group and the non-recurrent ITP group (n=50 and 122, respectively). The distribution of genotypes between the two groups was compared; however, no significant correlation was observed between the TLR7 rs3853839 genotypes and recurrence in pediatric patients with ITP (p>0.05, Table III). This indicates that the TLR7 rs3853839 polymorphism is not associated with ITP in relation to the recurrence of ITP disease.
The relationship between TLR7 rs3853839 C/G gene polymorphism and disease progression
All cases were classified into the chronic ITP group and the non-chronic ITP group (n=55 and 117, respectively). Among female ITP patients with different genotypes of TLR7 rs3853839, the proportion of GG genotype in chronic ITP patients was 16(57.14%), which was significantly higher than that in non-chronic ITP patients 17(31.48%), and the difference was statistically significant (χ2=8.968, p=0.011, Table III). Among male ITP patients with different genotypes of TLR7 rs3853839, the proportion of G/(-) genotype in chronic ITP patients was 22(81.48%), which was significantly higher than that in non-chronic ITP patients 37(58.73%), and the difference was statistically significant (χ2=4.333, p=0.037, Table III). The results showed that ITP patients with TLR7 rs3853839 GG genotype and G/(-) genotype had a significantly increased risk of developing chronic ITP.
Discussion
Recent information has shown that activation of TLR7 leads to increased levels of Th1 and Th17 cells, and their cytokines play a pivotal role in the pathogenesis of ITP.23,24 However, to the best of our knowledge, there is a lack of studies exploring the specific mechanisms involved. In this study, we aimed to clarify the characteristics of this mechanism associated with the TLR7 rs3853839 single nucleotide polymorphism. Our results indicate that the TLR7 rs3853839 GG genotype in female patients and the G/(-) genotype in male patients are linked to an increased risk of ITP, a more serious disease condition, and chronic disease progression. This finding is reported for the first time in the literature, as far as we are aware.
The pathogenesis of primary ITP involves multistep procedures, with genetic factors playing a pivotal role despite it being an autoimmune disease.25 The principal function of the TLR7 gene is to regulate the innate immune response.4,26 Excessive activation or impaired function of TLR7 may lead to innate immune dysfunction and the breakdown of immune tolerance, subsequently resulting in the onset of autoimmune diseases.27 The immune system homeostasis in ITP patients typically relies on the dynamic equilibrium between Th1 and Th2 cells. However, any disruption to this balance can potentially induce changes in ITP.11 Yang’s research indicates that the activation of TLR7 can lead to the polarization of Th1 immune cells, potentially influencing the development of ITP.28 TLR7 has also been found to recognize endogenous single-stranded RNA. When it is activated, a series of reactions occur through the classical myeloid cell differentiation factor 88 pathway, which promotes an increase in inflammatory factors such as IL-6, TNF-α, and IFN-γ, thus triggering the differentiation of Th17 cells and dendritic cells and ultimately leads to the immune inflammatory response.29,30 Previous research has indicated a notable increase in the quantities of Th17 cells and their associated cytokines, IL-6 and TNF-α, in patients with ITP.23,24 IL17 can induce the production of inflammatory cytokines such as IFN-γ, IL-1, and IL-6, further increasing the production of antiplatelet antibodies in mice3,31 and patients with ITP.32,33 Therefore, the TLR7 gene may cause ITP through these pathways, and its specific mechanism needs further study.
Rs3853839, located in the 3′ UTR of TLR7, has been proven to be functional and related to an increase in TLR7 mRNA and TLR7 protein expression and the stimulation of IFN gene upregulation to cause the occurrence of disease.18,34 The 3′ UTR is an essential regulatory region for expressing many genes that regulate mRNA translation, degradation, and subcellular localization by influencing RNA-binding proteins or noncoding RNAs.35 Raafat et al.36 reported that miRNAs could regulate TLR signaling through direct effects on expression or by modulation of downstream regulators, adaptor molecules, and cytokines. The TLR7 rs3853839 SNP can potentially impact gene expression by abolishing, weakening, or creating miRNA binding sites.37 The non-risk C allele of TLR7 rs3853839 matches a predicted binding site of microRNA-3148 (miR-3148), resulting in fast transcript breakdown and reducing TLR7 mRNA levels.38
TLR7 rs3853839 SNP in Non-Hematological Disorders
It has been reported that the TLR7 rs3853839 SNP is associated with human SLE.39 Azab et al.12 reported that the TLR7 rs3853839 GG genotype and G allele were significantly associated with the pathogenesis of SLE in Egyptian patients. Yue et al.18 reported that the TLR7 rs3853839 C allele polymorphism is sex-specific and has a protective effect on the persistence of hepatitis C prevention in Chinese women. Xi et al.19 revealed that the TLR7 rs3853839 C/G polymorphism may play a role in susceptibility to knee OA. Shen’s study conducted on patients with systemic lupus erythematosus revealed that individuals carrying the G mutant allele exhibited elevated TLR7 mRNA transcripts. Heterozygous participants also had elevated levels of TLR7 mRNA containing the G allele. These findings support the role of TLR7 rs3853839 SNP in regulating TLR7 mRNA expression.34 El-Hefnawy et al.40 indicated that the GG genotype of the TLR7 rs3853839 SNP may be a genetic risk factor for severe COVID-19 and adverse clinical outcomes. Elevated TLR7 mRNA expression in severe cases indicated its potential as a biomarker for COVID-19 prognosis. These findings also support the role of TLR7 rs3853839 SNP in regulating TLR7 mRNA expression in non-hematological disorders.
To our knowledge, this study represents the first demonstration of the association between TLR7 rs3853839 C/G SNP and the susceptibility and clinical manifestations of ITP within the Chinese Han population. We also observed that the GG and G/(-) genotypes are associated with an increased risk of ITP. The GG and G/(-) genotypes may result in heightened activation of TLR7, thus enhancing the self-reactivity of the immune system and increasing the risk of ITP. The GG genotype and G/(-) genotypes were shown to be substantially more common among severe patients in the current investigation. This phenomenon may be explained by excessive activation of TLR7, which may lead to a stronger inflammatory and autoimmune response, aggravating platelet destruction and production inhibition. This study also demonstrated that the GG and G/(-) genotypes may be predictive indicators for the chronic progression of ITP in pediatric patients. The continuous activation of TLR7 may lead to chronic disorders of the immune system, promoting the chronicity of ITP. This finding provides new insights into the individualized treatment of ITP.
Our study had certain limitations. Firstly, the participants included in our study were exclusively from the Chinese Han population. A more comprehensive investigation involving multi-racial populations is imperative to authenticate the correlations between TLR7 rs3853839 gene polymorphisms and ITP. Secondly, the biological functions of TLR7 rs3853839 remain unknown, and further mechanistic research is essential to elucidate the impact of TLR7 rs3853839 gene polymorphisms on the transcription, splicing, and translation efficiency of TLR7. The third and last limitation is that the time required to draw blood is not standard. Moreover, treatment modalities other than steroids could not be examined in this study. Despite this, we believe that our results accentuate the role of immunological derangements in its pathogenesis and will lead to further studies. The course of TLR7 mRNA individually in the same patient throughout the disease course and the cut-off levels at presentation, which predict the potential severity and progressivity of ITP in an individual patient, even at presentation, can be examined in further studies. These will enable individualized therapy in ITP, like avoidance of steroids but treatment with TLR7 inhibitors.
In conclusion, this study suggests TLR7 rs3853839 gene polymorphisms may be a potential genetic marker for ITP, affecting disease susceptibility, severity, and treatment response. These findings provide new insights into the pathogenesis of ITP and may provide a basis for developing personalized treatment strategies.
Ethical approval
The study was approved by Medical Ethics Committee of Linyi People’s Hospital (date: 10.26.2022, number: YX200343). Moreover, the participants’ families were informed about the study, and their written consent was obtained.
Source of funding
This study was funded by the Suzhou Science and Technology Development Plan Project, China (No. SYW2024004).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Madkhali MA. Recent advances in the management of immune thrombocytopenic purpura (ITP): a comprehensive review. Medicine (Baltimore) 2024; 103: e36936. https://doi.org/10.1097/MD.0000000000036936
- Kim DS. Recent advances in treatments of adult immune thrombocytopenia. Blood Res 2022; 57: 112-119. https://doi.org/10.5045/br.2022.2022038
- Semple JW, Rebetz J, Maouia A, Kapur R. An update on the pathophysiology of immune thrombocytopenia. Curr Opin Hematol 2020; 27: 423-429. https://doi.org/10.1097/MOH.0000000000000612
- Nomura S. Advances in diagnosis and treatments for immune thrombocytopenia. Clin Med Insights Blood Disord 2016; 9: 15-22. https://doi.org/10.4137/CMBD.S39643
- Narayanankutty A. Toll-like receptors as a novel therapeutic target for natural products against chronic diseases. Curr Drug Targets 2019; 20: 1068-1080. https://doi.org/10.2174/1389450120666190222181506
- Tărniceriu CC, Hurjui LL, Florea ID, et al. Immune thrombocytopenic purpura as a hemorrhagic versus thrombotic disease: an updated insight into pathophysiological mechanisms. Medicina (Kaunas) 2022; 58: 211. https://doi.org/10.3390/medicina58020211
- Jia YP, Wang K, Zhang ZJ, et al. TLR2/TLR4 activation induces tregs and suppresses intestinal inflammation caused by Fusobacterium nucleatum in vivo. PLoS One 2017; 12: e0186179. https://doi.org/10.1371/journal.pone.0186179
- Nyirenda MH, Sanvito L, Darlington PJ, et al. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol 2011; 187: 2278-2290. https://doi.org/10.4049/jimmunol.1003715
- Yu N, Weng Y, Liu W, et al. TLRs induce Th1/Th2 responses by affecting the secretion of CCL2 at the maternal-foetal interface. Int Immunopharmacol 2021; 100: 108070. https://doi.org/10.1016/j.intimp.2021.108070
- Kostic M, Zivkovic N, Cvetanovic A, Marjanović G. CD4+ T cell phenotypes in the pathogenesis of immune thrombocytopenia. Cell Immunol 2020; 351: 104096. https://doi.org/10.1016/j.cellimm.2020.104096
- Li Q, Liu Y, Wang X, et al. Regulation of Th1/Th2 and Th17/Treg by pDC/mDC imbalance in primary immune thrombocytopenia. Exp Biol Med (Maywood) 2021; 246: 1688-1697. https://doi.org/10.1177/15353702211009787
- Azab MM, Mostafa FM, Khalil M, et al. Association of TLR7 and TLR9 genes polymorphisms in Egyptian patients with systemic lupus erythematosus. Heliyon 2022; 8: e11680. https://doi.org/10.1016/j.heliyon.2022.e11680
- Sood R, Wong W, Jeng M, Zehnder JL. Gene expression profile of idiopathic thrombocytopenic purpura (ITP). Pediatr Blood Cancer 2006; 47: 675-677. https://doi.org/10.1002/pbc.20981
- Hesham M, Hassan T, Fawzy A, et al. PTPN22 gene polymorphism as a genetic risk factor for primary immune thrombocytopenia in Egyptian children. Expert Rev Hematol 2021; 14: 877-881. https://doi.org/10.1080/17474086.2020.1838895
- Liu Y, Wang Y, Zhang C, et al. HDAC3 single-nucleotide polymorphism rs2530223 is associated with increased susceptibility and severity of primary immune thrombocytopenia. Int J Lab Hematol 2022; 44: 875-882. https://doi.org/10.1111/ijlh.13857
- Zhang D, Zhang X, Li H, Xue F, Zhang L, Yang R. Association of FOXP3 gene polymorphisms with chronic immune thrombocytopenia in a Chinese Han population. Int J Lab Hematol 2021; 43: 1104-1109. https://doi.org/10.1111/ijlh.13525
- Wang T, Marken J, Chen J, et al. High TLR7 expression drives the expansion of CD19+CD24hiCD38hi transitional B cells and autoantibody production in SLE patients. Front Immunol 2019; 10: 1243. https://doi.org/10.3389/fimmu.2019.01243
- Yue M, Feng L, Tang SD, et al. Sex-specific association between X-linked Toll-like receptor 7 with the outcomes of hepatitis C virus infection. Gene 2014; 548: 244-250. https://doi.org/10.1016/j.gene.2014.07.040
- Xi X, Mehmood A, Niu P, et al. Association of X-linked TLR-7 gene polymorphism with the risk of knee osteoarthritis: a case-control study. Sci Rep 2022; 12: 7243. https://doi.org/10.1038/s41598-022-11296-4
- Inoue N, Katsumata Y, Watanabe M, et al. Polymorphisms and expression of toll-like receptors in autoimmune thyroid diseases. Autoimmunity 2017; 50: 182-191. https://doi.org/10.1080/08916934.2016.1261835
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv 2019; 3: 3780-3817. https://doi.org/10.1182/bloodadvances.2019000812
- Mehta B, Daniel R, Phillips C, McNevin D. Forensically relevant SNaPshot® assays for human DNA SNP analysis: a review. Int J Legal Med 2017; 131: 21-37. https://doi.org/10.1007/s00414-016-1490-5
- Feng X, Scheinberg P, Samsel L, et al. Decreased plasma cytokines are associated with low platelet counts in aplastic anemia and immune thrombocytopenic purpura. J Thromb Haemost 2012; 10: 1616-1623. https://doi.org/10.1111/j.1538-7836.2012.04757.x
- Rocha AM, Souza C, Rocha GA, et al. The levels of IL-17A and of the cytokines involved in Th17 cell commitment are increased in patients with chronic immune thrombocytopenia. Haematologica 2011; 96: 1560-1564. https://doi.org/10.3324/haematol.2011.046417
- Georgi JA, Middeke JM, Bornhäuser M, Matzdorff A, Trautmann-Grill K. Deciphering the genetic basis of immune thrombocytopenia: current evidence for genetic predisposition in adult ITP. Blood Adv 2023; 7: 3710-3724. https://doi.org/10.1182/bloodadvances.2023009949
- Liao WL, Wan L, Wang TY, et al. Association of TLR7 and TSHR copy number variation with Graves’ disease and Graves’ ophthalmopathy in Chinese population in Taiwan. BMC Ophthalmol 2014; 14: 15. https://doi.org/10.1186/1471-2415-14-15
- Wen L, Zhang B, Wu X, et al. Toll-like receptors 7 and 9 regulate the proliferation and differentiation of B cells in systemic lupus erythematosus. Front Immunol 2023; 14: 1093208. https://doi.org/10.3389/fimmu.2023.1093208
- Yang Q, Wang B, Yu H, et al. TLR7 promotes Th1 polarization in immune thrombocytopenia. Thromb Res 2011; 128: 237-242. https://doi.org/10.1016/j.thromres.2011.02.024
- Ye J, Wang Y, Liu X, et al. TLR7 Signaling regulates Th17 cells and autoimmunity: novel potential for autoimmune therapy. J Immunol 2017; 199: 941-954. https://doi.org/10.4049/jimmunol.1601890
- Patra MC, Shah M, Choi S. Toll-like receptor-induced cytokines as immunotherapeutic targets in cancers and autoimmune diseases. Semin Cancer Biol 2020; 64: 61-82. https://doi.org/10.1016/j.semcancer.2019.05.002
- Yoh K, Morito N, Ojima M, et al. Overexpression of RORγt under control of the CD2 promoter induces polyclonal plasmacytosis and autoantibody production in transgenic mice. Eur J Immunol 2012; 42: 1999-2009. https://doi.org/10.1002/eji.201142250
- Cao J, Chen C, Li L, et al. Effects of high-dose dexamethasone on regulating interleukin-22 production and correcting Th1 and Th22 polarization in immune thrombocytopenia. J Clin Immunol 2012; 32: 523-529. https://doi.org/10.1007/s10875-012-9649-4
- Hassan T, Zakaria M, Diaa A, et al. Contribution of T helper 17 cells and interleukin-17 to the pathogenesis of primary immune thrombocytopenia in Egyptian children. Eur J Pediatr 2023; 182: 5673-5679. https://doi.org/10.1007/s00431-023-05242-3
- Shen N, Fu Q, Deng Y, et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A 2010; 107: 15838-15843. https://doi.org/10.1073/pnas.1001337107
- Mitschka S, Mayr C. Context-specific regulation and function of mRNA alternative polyadenylation. Nat Rev Mol Cell Biol 2022; 23: 779-796. https://doi.org/10.1038/s41580-022-00507-5
- Raafat II, El Guindy N, Shahin RMH, Samy LA, El Refai RM. Toll-like receptor 7 gene single nucleotide polymorphisms and the risk for systemic lupus erythematosus: a case-control study. Z Rheumatol 2018; 77: 416-420. https://doi.org/10.1007/s00393-017-0283-7
- Karthi S, Rajeshwari M, Francis A, et al. 3’-UTR SNP rs2229611 in G6PC1 affects mRNA stability, expression and glycogen storage disease type-ia risk. Clin Chim Acta 2017; 471: 46-54. https://doi.org/10.1016/j.cca.2017.05.016
- Deng Y, Zhao J, Sakurai D, et al. MicroRNA-3148 modulates allelic expression of toll-like receptor 7 variant associated with systemic lupus erythematosus. PLoS Genet 2013; 9: e1003336. https://doi.org/10.1371/journal.pgen.1003336
- Li S, Di D, Wu X, et al. Association study between X-linked susceptibility genes and clinical features in Chinese female patients with systemic lupus erythematosus. Autoimmunity 2019; 52: 289-293. https://doi.org/10.1080/08916934.2019.1688792
- El-Hefnawy SM, Eid HA, Mostafa RG, Soliman SS, Omar TA, Azmy RM. COVID-19 susceptibility, severity, clinical outcome and Toll-like receptor (7) mRNA expression driven by TLR7 gene polymorphism (rs3853839) in middle-aged individuals without previous comorbidities. Gene Rep 2022; 27: 101612. https://doi.org/10.1016/j.genrep.2022.101612
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















