Graphical Abstract
Abstract
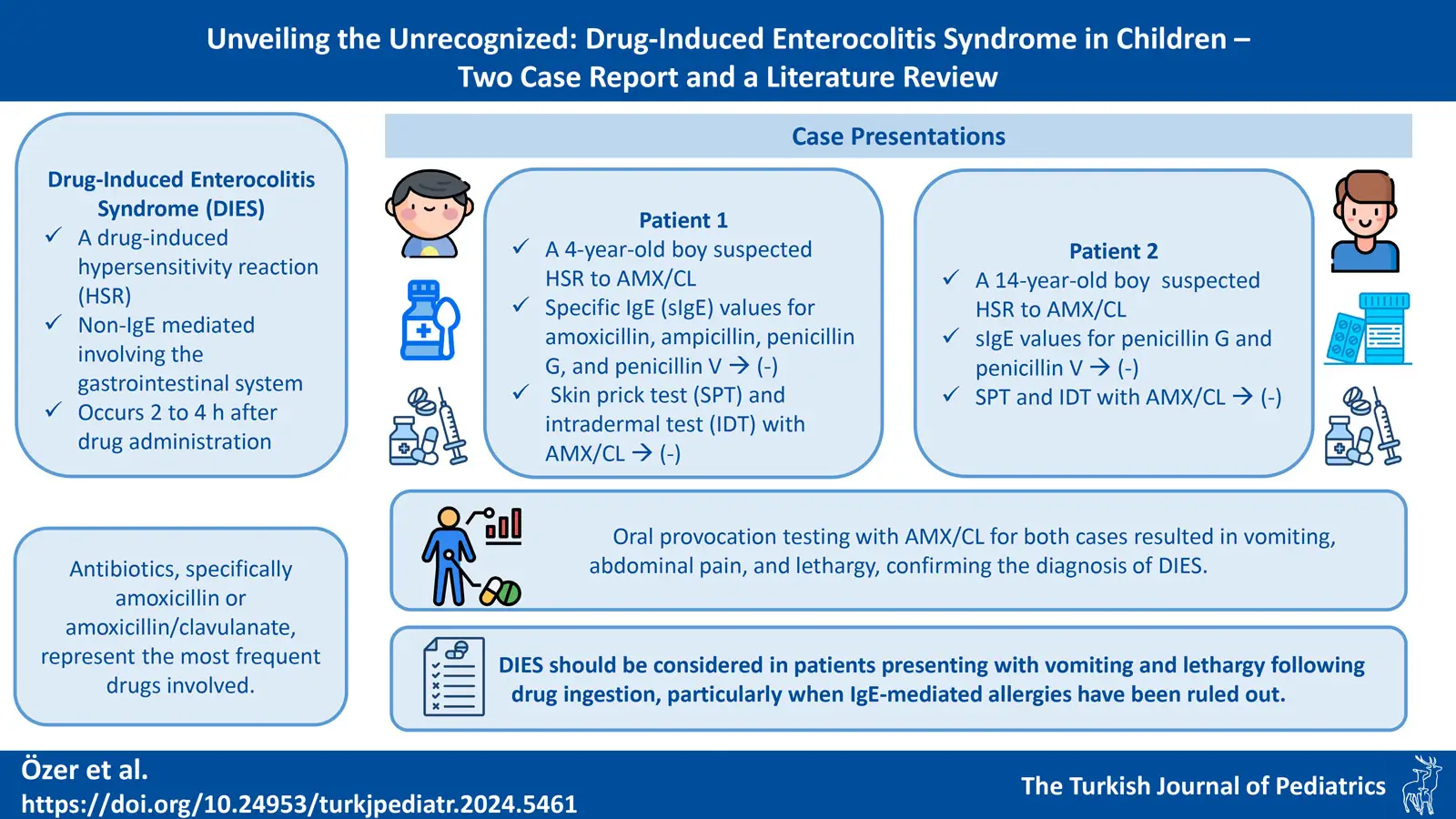
Background. Drug-induced enterocolitis syndrome (DIES) is a recently defined clinical entity, first described in 2014. DIES is a hypersensitivity reaction with non-IgE mechanisms involving the gastrointestinal tract, occurring 1 to 4 hours after drug ingestion. Antibiotics are most commonly responsible, particularly amoxicillin or amoxicillin / clavulanic acid (AMX/CL). The main criterion is recurrent, often uncontrollable vomiting occurring 1-4 hours after drug ingestion, without classic IgE-mediated allergic symptoms such as cutaneous or respiratory reactions. To the best of our knowledge, 10 pediatric cases of DIES have been described in the literature.
Case Presentations. A 4-year-old male and a 14-year-old male presented to our pediatric allergy clinic with a suspected hypersensitivity reaction to AMX/CL, and their specific IgE tests for penicillin G and penicillin V were negative. The younger patient was also tested for specific IgE against amoxicillin and ampicillin, which were also negative. Skin prick tests and intradermal test with AMX/CL were negative in both patients, but oral provocation testing with AMX/CL resulted in abdominal pain, vomiting and lethargy, confirming the diagnosis of DIES.
Conclusions. DIES should be considered in patients presenting with vomiting and lethargy following drug ingestion, particularly when IgE-mediated allergies have been ruled out. Early recognition and appropriate management, including drug provocation testing in a controlled setting, are crucial to ensure optimal patient outcomes. By presenting these two rare cases, we aim to raise awareness and deepen the understanding of DIES among healthcare professionals, which could contribute to earlier diagnosis and better patient outcomes.
Keywords: amoxicillin/clavulanic acid, children, drug-induced enterocolitis syndrome, rare allergic diseases
Introduction
Drug hypersensitivity reactions (DHRs) are increasing in both adult and pediatric populations, especially in recent years. While IgE-mediated mechanisms are the most common cause of DHRs, non-IgE-mediated reactions involving drug-specific T-cell responses can also occur.1
Drug-induced enterocolitis syndrome (DIES) is a non-IgE-mediated, drug-specific T-cell-mediated hypersensitivity reaction, primarily affecting the gastrointestinal system. The syndrome typically manifests 1 to 4 hours after drug ingestion and is characterized by symptoms such as nausea, vomiting, abdominal pain, diarrhea, pallor, lethargy, and dehydration. Hypovolemic shock has been reported in some cases.2
The non-immediate adverse drug reaction known as DIES is not well understood. It is very important that this clinical picture, whose pathogenic mechanism is still unclear, is recognized early and treated correctly by physicians.2,3 We present two pediatric cases to raise awareness of this rare clinical entity, first described in 2014.
Case Presentations
Patient 1
A 4-year-old boy was admitted to our pediatric allergy outpatient clinic with a suspected hypersensitivity reaction (HSR). At 15 months of age, he developed maculopapular exanthema on the third day of treatment with amoxicillin / clavulanic acid (AMX/CL), prompting discontinuation of the medication. The rash disappeared within 2-3 days. The patient had not used AMX/CL again since the initial reaction.
The patient was evaluated in our pediatric allergy clinic and amoxicillin, ampicillin, penicillin G, and penicillin V specific IgE (sIgE) tests were negative. Skin prick test (SPT) and intradermal test (IDT) with AMX/CL (20 mg/mL) were negative. With his family’s informed consent, the patient was set up for an open drug provocation test (DPT). The AMX/CL oral suspension was divided into two doses, each representing a portion between 10-90% of the total therapeutic dose and administered at 30-minute intervals (reaching a total of 40 mg/kg/day).
One and a half hours after the last dose, the patient developed severe abdominal pain, four episodes of intermittent severe vomiting, pallor, and lethargy, without cutaneous, mucosal, respiratory, or systemic symptoms. Vital signs revealed an oxygen saturation of 98%, respiratory rate of 22/min, blood pressure of 80/50 mmHg, and heart rate of 105/min.
Intravenous (IV) 20 mL/kg 0.9% NaCI and ondansetron were administered. Laboratory tests, obtained three hours after the onset of vomiting, showed white blood cells (WBC): 12.98x109/L (normal range 5.5-15.5), neutrophils: 9.15x109/L (normal range 1.5-8.5), platelets (PLT): 401x109 /L (normal range 300±50), C-reactive protein (CRP): 0.3 mg/L (<5 mg/L), blood urea nitrogen (BUN): 30 mg/dL (0-23), creatinine: 0.69 mg/dL (0.3-1.2); and normal serum electrolytes. Acidosis was detected in blood gas analysis; pH: 7.29 (7.35-7.45), HCO3: 18.6 mmol/L (18-23), PCO2: 38 mmHg (32-46). The patient was closely monitored, and lethargy improved after 12 hours. After 24 hours of observation, oral intake improved. On the day of the challenge test, there were no confounding variables or infectious contexts. No mast cell degranulation was observed (tryptase levels: 3.78 ug/L [normal: <11.4]) three hours after reaction). The patient was diagnosed with DIES, fulfilling the diagnostic criteria (Table I).3 Afterwards, the patient was evaluated for beta-lactam cross-reactivity, and he tolerated cefuroxime without any problem.
| DIES: Drug-induced enterocolitis syndrome. |
| Table I. Diagnostic Criteria for Patients Presenting with Possible DIES. |
| Major criterion |
| 1. Vomiting in the 1- to 4-h period after ingestion of the suspected drug and absence of classic IgE-mediated allergic skin or respiratory symptoms |
| Minor criteria |
|
1. A second episode of repetitive vomiting after ingestion of the same drug 2. Repetitive vomiting episode 1-4 h after ingestion of a different drug 3. Extreme lethargy 4. Marked pallor 5. Need for emergency department visit 6. Need for intravenous fluid support 7. Diarrhea in 24 h (usually 5-10 hours) after ingested drug 8. Hypotension 9. Hypothermia |
Patient 2
A 14-year-old male patient was referred to our pediatric allergy outpatient clinic for recurrent vomiting episodes starting one hour after AMX/CL ingestion at 3 and 11 years of age. No other AMX/CL exposures were reported.
Specific IgE tests for penicillin G and V were negative. SPT followed by IDT were performed using AMX/CL (20 mg/mL), and both were negative. The patient was scheduled to undergo an open DPT with informed consent from his family. The AMX/CL tablet was administered in three doses, each representing 10-40-50% of the total therapeutic dose, at 30-minute intervals (reaching a total of 40 mg/kg/day). One hour and 15 minutes after the last dose, the patient developed severe abdominal pain, three episodes of severe vomiting, lethargy, and pallor. IV hydration with 0.9% NaCl, methylprednisolone, and ondansetron were administered. There were no cutaneous or respiratory system findings other than vomiting. Upon assessment of vital signs, oxygen saturation was 97%, respiratory rate was 20 breaths per minute, blood pressure was 100/60 mmHg, and heart rate was 95 beats per minute. Laboratory results revealed leukocytosis (WBC: 13.34x109/L [normal range 4.5-13]) and neutrophilia (12.53x109/L [normal range 1.8-8]), PLT: 314x109/L (normal range 300±50), BUN: 11 mg/dL (normal range 0-23), creatinine: 0.51 mg/dL (normal range 0.3-1.2); and serum electrolytes were within normal physiologic range. Tryptase levels were normal (0.98 µg/L). On the day of the challenge test, there were no signs of infection or any other potential confounding factors. The patient, who met the diagnostic criteria, was diagnosed with DIES (Table I).2 SPT/IDT and DPT with cefuroxime were planned for alternative beta-lactam antibiotics.
Discussion
Drug-induced enterocolitis syndrome is a drug-induced non-IgE mediated HSR, primarily affecting the gastrointestinal system and typically occurring 1 to 4 h after drug administration. This clinical picture, which can cause severe clinical manifestations such as lethargy, hypotension and hypovolemic shock, is poorly recognized worldwide.4 Timely diagnosis and appropriate management of DIES are crucial. In this paper, we present two cases of DIES to highlight the need for increased awareness of this condition.
In 2014, an Italian research group was the first to describe a case of a 6-year-old girl who developed vomiting 1-2 hours after AMX ingestion. They coined the term DIES to describe this clinical picture, as its clinical presentation and laboratory findings were reminiscent of food protein-induced enterocolitis syndrome (FPIES). The immunologic mechanisms underlying DIES are still not fully understood. It has been suggested that metabolites formed after the drug passes through hepatic and intestinal processes may cause drug-induced intestinal damage.2 Reports of DIES cases were published from Spain in 2017,5 Netherlands in 20193, and most recently from Türkiye in 2024.6 The fact that all of the 10 pediatric cases described were from European countries suggests that awareness of DIES is very low worldwide (Table II).3-10 The incidence is unknown, but one Italian children’s hospital reported a 0.4% prevalence of DIES among pediatric patients referred for suspected DHRs.8 The low number of reported cases suggests that DIES may be underdiagnosed, particularly outside Europe.
|
* Review of the published pediatric cases according to the last search of PubMed in September 2024 (The case described from Türkiye is not available on PubMed) AMX: Amoxicillin, AMX/CL: Amoxicillin/clavulanate, DPT: Drug provocation test, SPT: Skin prick tests, IDT: Intradermal tests, sIgE: Serum-specific IgE, n.s: Not specified, n.a: Not applied, Ref: Reference |
|||||||||
| Table II. Review of the published cases according to the last search of PubMed in September 2024*. | |||||||||
| Age (yr), sex, country |
Triggering Drug | History of drug reaction | DPT | Suspicious Drug Laboratory Findings | Ref. | ||||
| Latency, hr | Signs/Symptoms | Treatment | Laboratory Findings | SpIgE | SPT and IDT | ||||
| 6, F, Italy | AMX |
1. Vomiting 2. Morbilliform rash (one day later) |
2 |
1. Vomiting 2. Diarrhea 3. Pallor 4. Lethargy 5. Hypotension |
1. Saline solution infusion 2. Steroid |
1. Leukocytosis 2. Neutrophilia |
Negative | Negative | 10 |
| 3, M, Spain | AMX | 1. Urticaria | 4 |
1. Vomiting 2. Diarrhea 3. Abdominal pain |
1. Saline solution infusion 2. Steroid 3. Antiemetics |
1. Leukocytosis 2. Neutrophilia |
Negative | Negative | 5 |
| 4, M, Netherlands | AMX |
1. Erythematous skin reaction (When 2 years old) 2. Vomiting (When 4 years old) 3. Lethargy (When 2 years old) |
1.5 |
1. Vomiting 2. Pale 3. Abdominal pain 4. Lethargy |
1. Adrenalin 2. Steroid 3. Ondansetron |
1. Leukocytosis 2. Neutrophilia |
Negative |
Negative (only SPT) IDT was not performed |
3 |
| 10, F, France |
AMX |
1. Vomiting 2. Pallor 3. Diarrhea |
3 |
1. Vomiting 2. Pallor 3. Abdominal pain 4. Lethargy 5. Diarrhea |
1. Only rehydrated orally | n.s | n.a | n.a | 7 |
| 6, M, Italy | AMX/CL | 1. Angioedema | 2.5 |
1. Vomiting 2. Pale 3. Lethargy |
1. Saline solution infusion |
1. Leukocytosis 2. Neutrophilia |
Negative | Negative | 8 |
| 14, F, Italy | AMX/CL |
1. Vomiting 2. Lethargy |
2.5 |
1. Vomiting 2. Pallor 3. Lethargy 4. Abdominal pain 5. Malaise |
1. Saline solution infusion 2. Ondansetron |
1. Leukocytosis 2. Neutrophilia |
n.a | Only IDT | 8 |
| 9, F, Switzerland | AMX/CL |
1. Maculopapular exanthema |
3 |
1. Vomiting 2. Pallor 3. Lethargy |
n.s | n.s | n.a | Only IDT | 8 |
| 0.8, M, France | Paracetamol |
1. Vomiting 2. Asthenia 3. Pallor 4. Tachycardia |
4 |
1. Vomiting 2. Pallor 3. Lethargy |
1. Rehydration 2. Corticosteroid |
n.s | Negative | Negative | 9 |
| 4, M, France |
AMX |
1. Progressively extensive erythematous rash 2. Eyelid edema (On the seventh day of treatment) |
2 |
1. Vomiting 2. Abdominal pain 3. Pallor 4. Tachycardia |
1. Adrenalin 2. Saline solution infusion 3. Ondansetron |
n.s | n.a |
Negative (Only IDT) |
4 |
| 7, M, Türkiye | AMX/CL |
1. Vomiting 2. Lethargy |
2.5 |
1. Abdominal pain 2. Vomiting 4. Lethargy 5. Pallor |
1. Saline solution infusion |
1. Neutrophilia | n.a | n.a | 6 |
The primary diagnostic challenge in DIES lies in differentiating it from IgE-mediated hypersensitivity reactions. The absence of skin and respiratory symptoms, along with normal tryptase levels, are the most critical points in differentiating it from an IgE-mediated reaction.1,2 Upon evaluating the clinical findings following DPT in 10 cases from the literature and 2 cases presented by us, vomiting was observed in all cases (12/12 patients). Pallor (11/12 patients) and lethargy (10/12 patients) were the other most common findings. Vomiting after drug ingestion may develop both with an IgE-mediated mechanism and with a non-IgE-mediated mechanism (as in DIES). The key distinguishing factor is the timing of vomiting onset following drug ingestion in DIES, it begins 1.5 to 2 hours after ingestion, whereas in IgE-mediated reactions, it occurs within the first hour of exposure.2
Among the pediatric DIES cases in the literature, five were caused by AMX, 4 by AMX/CL, and 1 by paracetamol. The two cases we presented were AMX/CL-induced, which is consistent with previous reports.2 Nausea, vomiting, and diarrhea are reported to be common side effects of AMX.11 For these reasons, in patients who develop nausea, vomiting, and diarrhea after AMX, DPT should be performed under hospital conditions to diagnose DIES.
Diagnostic criteria for DIES were proposed by Van Thuijl et al. (Table I).3 For the diagnosis, 1 major and 3 minor criteria must be met (in addition, IgE-mediated cutaneous and respiratory symptoms must be absent). As in the 10 cases described in the literature, these diagnostic criteria were met in the two cases we presented.
There are no specific or diagnostic laboratory parameters for DIES. There is no increase in tryptase levels. The most notable laboratory finding is “neutrophilic leukocytosis”. The neutrophil peak in DIES is expected approximately 6 hours after drug ingestion due to increased IL-8 and cortisol levels.2 In the described cases of DIES, there is no information about the time of collection of blood tests. Leukocytosis and neutrophilia were described in 6 cases in the literature, while in other 4 cases, no information about these tests was provided. In the first case we presented, blood tests were taken 3 hours after drug intake, and although leukocytosis was absent, neutrophilia was present. In the second case, blood tests were taken 6 hours after drug ingestion, and marked leukocytosis and neutrophilia were detected. To support the diagnosis, obtaining a complete blood count 6 hours after drug intake may be useful in confirming DIES, as this timing correlates with the characteristic laboratory findings.
In the cases we have presented, type A reactions are the most likely to be confused with DIES. Isolated gastrointestinal symptoms, such as vomiting, abdominal pain, and diarrhea, can occur as side effects after the use of certain medications. These symptoms are typically mild and transient, leading to their interpretation as pharmacological reactions.11 However, a key distinction between DIES and drug side effects lies in the severity, timing of onset, and the presence of associated systemic symptoms. In DIES, gastrointestinal symptoms are more severe, with vomiting often leading to significant dehydration and accompanied by systemic symptoms such as lethargy. This contrasts with the mild and self-limiting nature of typical drug side effects. Additionally, DIES symptoms usually begin 1 to 4 hours after drug ingestion, which represents a delayed onset compared to the immediate onset typically seen with drug side effects.2 Severe gastrointestinal distress, along with systemic symptoms like pallor and lethargy, suggests DIES rather than a simple drug side effect. Moreover, once IgE-mediated reactions (e.g., anaphylaxis) have been ruled out, DIES should be considered in the differential diagnosis.
Patch tests are used in the evaluation of delayed-type hypersensitivity reactions; however, their sensitivity is limited in reactions associated with the gastrointestinal system.12,13 In the majority of DIES cases reported in the literature, patch testing was not performed. Similarly, in our study, the diagnosis was confirmed based on clinical findings and DPT. Patch testing presents practical challenges in pediatric patients and may not be adequate for assessing delayed reactions in the gastrointestinal tract, particularly in systemic conditions like DIES. However, we think that whether patch tests play a role in understanding DIES pathophysiology may be investigated in future studies.
The management of DIES is primarily supportive care. Since the most prominent clinical finding in the acute phase of DIES is vomiting and the resulting fluid loss, treatment is mainly focused on addressing these symptoms.2 In nearly all cases reported in the literature, IV 0.9% NaCl and IV ondansetron were administered, leading to clinical improvement. Both IV 0.9% NaCl and IV ondansetron were also administered in the cases we presented. While the efficacy of corticosteroids has not been proven, they may be considered in patients with severe symptoms, as cell-mediated inflammation is suspected in FPIES.2 We administered IV steroids in our second case. Adrenaline is not recommended in the management of DIES, which shares a similar pathophysiology with FPIES.2 However, intramuscular adrenaline was given in the two cases from the literature because it was difficult to distinguish DIES from anaphylaxis. Despite adrenaline administration, vomiting persisted in both patients and only resolved after ondansetron was given.3,4
In conclusion, the identification of all reported cases of DIES in Europe underscores the need for increased global awareness of this condition. DIES should be considered in patients presenting with vomiting and lethargy following drug ingestion, particularly when IgE-mediated allergies have been ruled out. Early recognition and appropriate management, including DPT in a controlled setting, are crucial to ensuring optimal patient outcomes. We hope that by presenting these two rare cases, we contribute to greater awareness and understanding of DIES.
Ethical approval
All the studies were performed by the Declaration of Helsinki and guidelines for good clinical practice. All legal representatives of the patients were informed, and their informed consent was obtained.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy 2019; 74: 1457-1471. https://doi.org/10.1111/all.13765
- Di Filippo P, Venanzi A, Ciarelli F, et al. Drug-induced enterocolitis syndrome in children. Int J Mol Sci 2023; 24: 7880. https://doi.org/10.3390/ijms24097880
- Van Thuijl AO, Landzaat LJ, Liem O, Emons JA, Arends NJ. Drug-induced enterocolitis syndrome (DIES): a clinical entity that deserves more awareness. Ann Allergy Asthma Immunol 2019; 122: 538-539. https://doi.org/10.1016/j.anai.2019.02.004
- Eyraud C, Biermé P, Adam M, Braun C. Drug-induced enterocolitis syndrome: a rare, severe, non-IgE-mediated immediate drug allergy. Case report and literature review. Arch Pediatr 2023; 30: 67-70. https://doi.org/10.1016/j.arcped.2022.11.007
- Infante S, Zapatero L. Drug-induced enterocolitis syndrome by amoxicillin. Pediatr Allergy Immunol 2017; 28: 105-106. https://doi.org/10.1111/pai.12643
- Yorusun G, Selmanoglu A, Sengul Emeksiz Z, Dibek Misirlioglu E. Drug-induced enterocolitis syndrome with amoxicillin/clavunate and safe alternative beta lactam antibiotic. Asthma Allergy Immunol 2024; 22: 329-332. https://doi.org/10.21911/aai.2024.578
- Worcel J, Tarelho M, Baron M, et al. Drug-induced enterocolitis syndrome (DIES) in a 10-year-old girl. Arch Pediatr 2020; 27: 51-52. https://doi.org/10.1016/j.arcped.2019.11.005
- Mori F, Liccioli G, Fuchs O, et al. Drug-induced enterocolitis syndrome: similarities and differences compared with food protein-induced enterocolitis syndrome. Pediatr Allergy Immunol 2021; 32: 1165-1172. https://doi.org/10.1111/pai.13491
- Pascal B, Evrard B, Merlin E, Egron C, Bonnet B, Michaud E. Drug-induced enterocolitis syndrome with paracetamol (acetaminophen) in a 12-month-old boy. Pediatr Allergy Immunol 2022; 33: e13755. https://doi.org/10.1111/pai.13755
- Novembre E, Mori F, Barni S, Pucci N. Drug-induced enterocolitis syndrome (DIES). Pediatr Allergy Immunol 2014; 25: 415-416. https://doi.org/10.1111/pai.12225
- Grattagliano I, Ubaldi E, Portincasa P. Drug-induced enterocolitis: prevention and management in primary care. J Dig Dis 2018; 19: 127-135. https://doi.org/10.1111/1751-2980.12585
- Cuomo B, Anania C, D'Auria E, et al. The role of the atopy patch test in the diagnostic work-up of non-IgE gastrointestinal food allergy in children: a systematic review. Eur J Pediatr 2023; 182: 3419-3431. https://doi.org/10.1007/s00431-023-04994-2
- Hassoun-Kheir N, Bergman R, Weltfriend S. The use of patch tests in the diagnosis of delayed hypersensitivity drug eruptions. Int J Dermatol 2016; 55: 1219-1224. https://doi.org/10.1111/ijd.13306
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















