Graphical Abstract

Abstract
Objective. We aimed to identify and compare systemic juvenile idiopathic arthritis (sJIA) patients receiving treatment with either glucocorticoids and/or conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) or biologic drugs.
Methods. This was a retrospective cross-sectional study. sJIA patients (n=138) were categorized into two groups: Group A (n=51) consisted of individuals who received only glucocorticoids and/or csDMARDs, while Group B (n=87) included those who received at least one biologic drug.
Results. Group B patients exhibited a higher prevalence of macrophage activation syndrome (MAS) (p=0.001) at presentation. C-reactive protein (CRP) levels and systemic Juvenile Arthritis Disease Activity Scores (sJADAS) at diagnosis were significantly higher in Group B (p<0.001). A higher proportion of Group B were able to discontinue glucocorticoid treatment in a shorter timeframe (p<0.001), and a higher number of patients in this group successfully discontinued glucocorticoids within the first year (p<0.001). Presentation with MAS (odds ratio [OR] 3.419, 95% confidence interval [CI] 1.194-9.792; p=0.022), polycyclic disease course (OR 4.351, 95% CI 1.329-14.240; p=0.015), CRP levels >13.6 mg/dL (OR 2.838, 95% CI 1.182-6.815; p=0.020) and sJADAS >24.1 (OR 4.490, 95% CI 1.725-11.684; p=0.002) at diagnosis were independent predictors of biologic requirement in treatment.
Conclusion. Patients with a history of MAS, polycyclic disease course, elevated CRP, and high sJADAS at diagnosis may require biologic drugs in the treatment. This observation could help clinicians tailor treatment according to the individual needs of sJIA patients.
Keywords: biologic drugs, disease-modifying antirheumatic drugs, glucocorticoids, systemic juvenile idiopathic arthritis
Introduction
Systemic juvenile idiopathic arthritis (sJIA) is a subtype of JIA characterized by arthritis as well as systemic symptoms that can affect various organs and systems.1 The sJIA treatment aims at reducing inflammation, relieving symptoms, and preventing complications. sJIA treatment may include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), and in some cases, biologic drugs.2,3
In the past, high-dose glucocorticoids were the first choice for the treatment of sJIA to suppress the cytokine storm.4,5 Recently, treatment strategies such as targeted therapy and early aggressive use of biologics have been available for these patients and have improved outcomes for patients with sJIA. The American College of Rheumatology (ACR) recommends csDMARDs or biologics following systemic glucocorticoids in patients with glucocorticoid resistance or severe systemic and joint findings.6 The choice between conventional therapy (glucocorticoids ± csDMARD) and biologic therapies depends on the severity of the disease and the response to treatment.7 In many cases, a step-up approach is employed.8
In this study, we aimed to compare patients using biologic drugs with patients on glucocorticoids ± csDMARD among patients with SJIA and identify associated factors for the treatment with biologic drugs.
Materials and Methods
This is a retrospective cross-sectional study. It was approved by the ethics committee of Hacettepe University (date: 15.06.2021, number: 2021-12). Informed consent was acquired from both parents and patients before they participated in the study. The study adhered to the ethical principles laid out in the 1964 Declaration of Helsinki and its subsequent revisions.
Patients
All patients with sJIA from May 2011 to June 2023 were included in the study. The patients before the biologic treatment era were excluded from the study. All participants fulfilled the ILAR classification criteria for sJIA.9 Demographics, clinical and laboratory features, disease courses, treatments, and outcomes of all patients were evaluated. Additionally, the systemic Juvenile Arthritis Disease Activity Score (sJADAS) was calculated at diagnosis and last visit.10 Disease courses were defined as monocyclic (only one-time flare lasting up to 24 months), polycyclic (with multiple flares separated by inactive periods), or persistent (marked by unceasing inflammation and progressive arthritis, often affecting multiple joints).11 For the clinically inactive disease, the following ACR definition was used: no active arthritis, a physician’s global assessment of disease activity score of 0, normal levels of erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP), the absence of sJIA features (fever, rash, serositis, splenomegaly, or generalized lymphadenopathy), the absence of uveitis, and duration of morning stiffness lasting less than 15 minutes.12 Remission was evaluated as on-drug and off-drug, and considered to be a clinically inactive disease for at least six months.
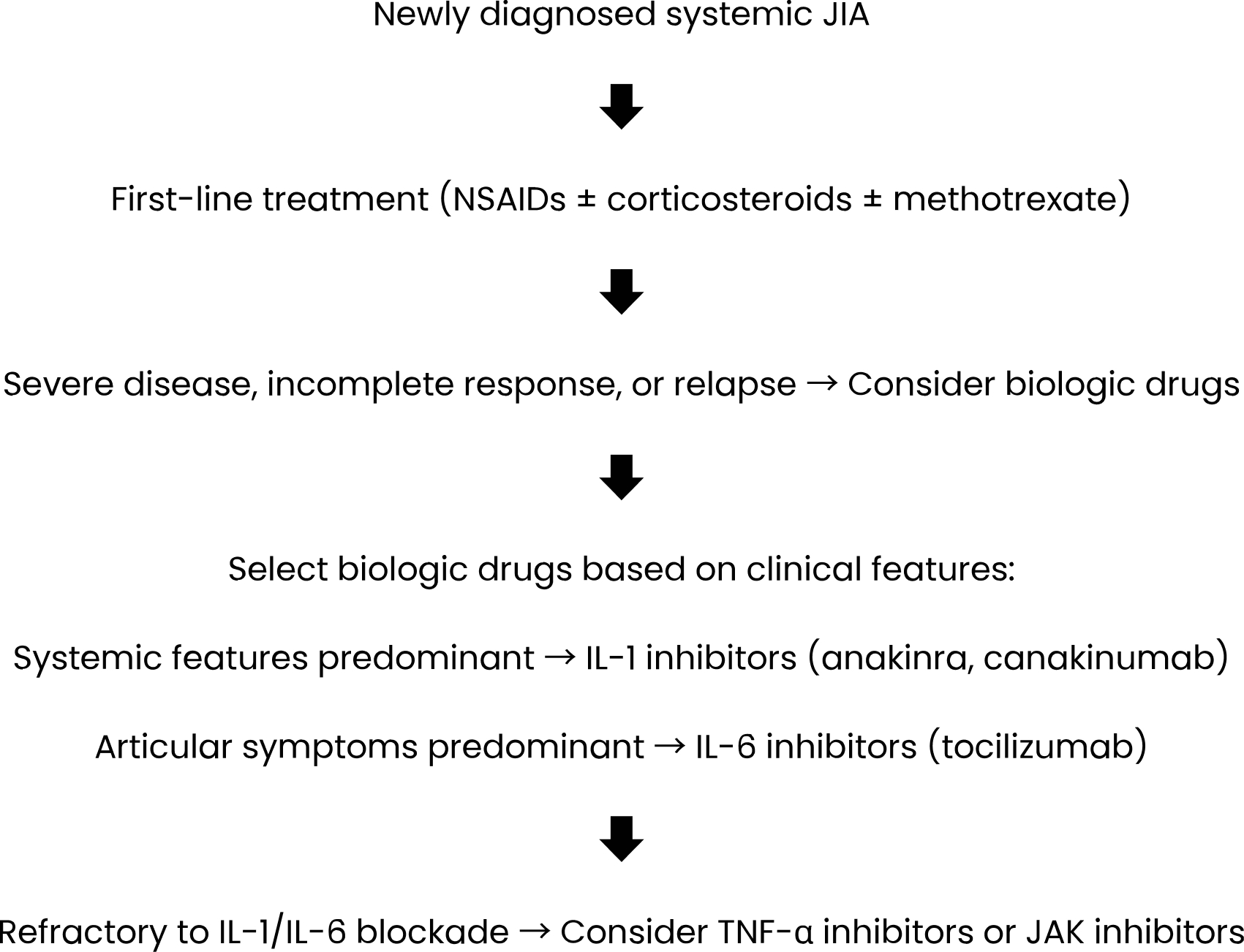
All patients were initially administered high doses of pulse intravenous glucocorticoids (10-30 mg/kg/day) for three days, and treatment was continued with oral glucocorticoids at 1-2 mg/kg/day in the follow-up. Methotrexate (MTX, 15-20 mg/m2/week subcutaneously) or cyclosporine-A (Cyc-A, 3-5 mg/kg/day orally) was frequently used as csDMARDs. In cases of severe disease, incomplete response, or relapse, biologic drugs were added (Fig. 1). The patients were divided into two groups: those who received glucocorticoids and/or csDMARDs alone (Group A) and those who received at least one biologic drug (Group B). Differences between these two groups were analyzed. Factors determining the requirement for the biologic treatment were identified.
Statistical analysis
To assess variable distribution, both visual methods (histograms and probability graphs) and analytical tests (Shapiro-Wilks) were utilized. The descriptive statistics were presented as frequency (n) and percentage (%) for categorical variables and as median (25th percentile [Q1] and 75th percentile [Q3]) for continuous variables. Categorical variables were compared using the chi-square test or Fisher’s exact test, while non-normally distributed continuous variables were compared using the Mann-Whitney U-test. Univariate analysis was employed to identify predictors of the requirement for biologic therapy. Continuous variables were dichotomized through ROC analysis, and variables with an unadjusted p-value below 0.05 in the logistic regression analysis were identified as potential predictive markers and included in the full model. The model was subsequently refined through multivariate logistic regression analyses during retrospective elimination. Significance was established at a p-value below 0.05, with a 95% confidence interval (CI) applied.
Results
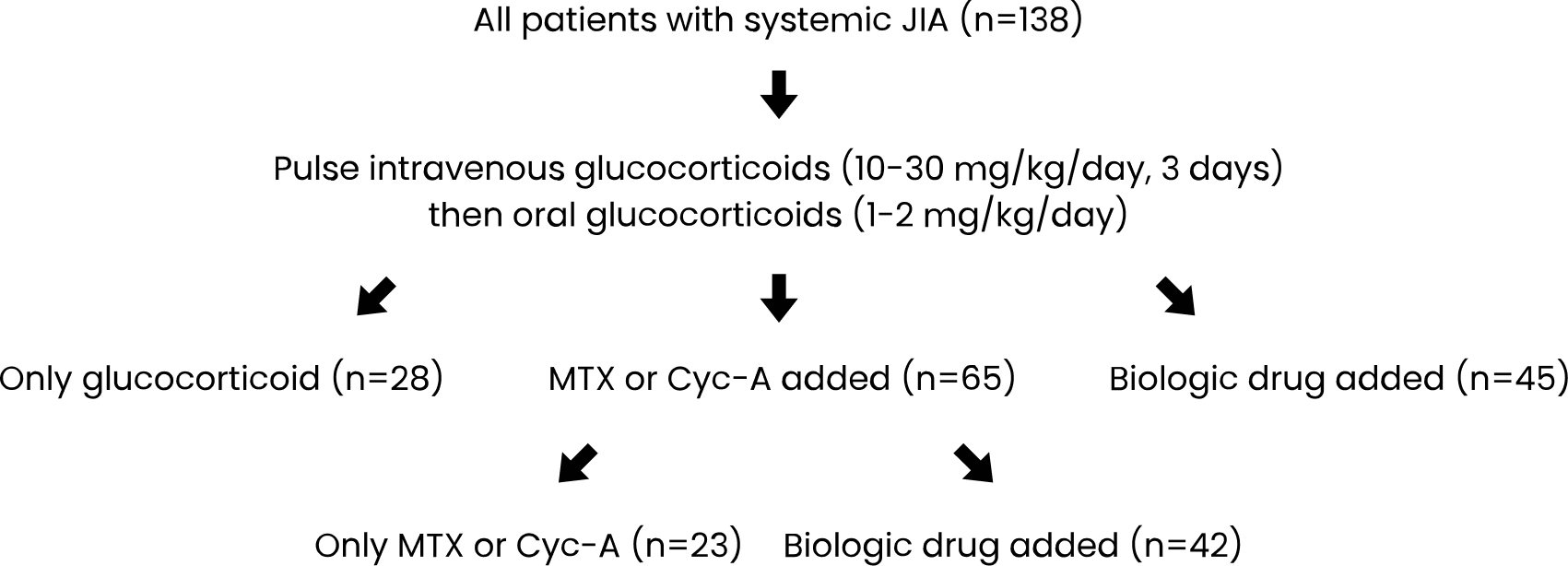
A total of 138 patients with sJIA were included in the study (F/M=0.8). The median age of the patients at diagnosis was 5.5 (1.9-11.6) years. In the initial treatment, after glucocorticoids, 65 patients (47.1%) were administered csDMARDs and 45 patients (32.6%) received biologic drugs (Fig. 2). In the median third month of the csDMARD therapy, a biologic agent was added to the treatment, if the disease was not clinically inactive. Therefore, 42 of 65 patients (64.6%) receiving csDMARDs were switched to biologic drugs. Twenty-eight patients (20.3%) received only glucocorticoids, while 23 patients (16.7%) received only csDMARDs (Fig. 2).
CsDMARDs were initiated for various reasons such as prominent articular symptoms (n=22), macrophage activation syndrome (MAS, n=12), and systemic symptoms that could not be controlled with glucocorticoids (n=11) at disease onset. Interleukin (IL)-1 inhibitors were used in 45 patients who had severe systemic symptoms and/or presented with MAS at disease onset. Anakinra was initiated in most of them, then it was switched to canakinumab. In 29 patients using csDMARDs, biologics were introduced due to a persistent polyarticular disease course. On the other hand, for 13 patients, the reason for initiating biologics was the resistant/recurrent systemic inflammation. Notably, all patients with persistent polyarticular course were treated with tocilizumab.
As a result, 51 patients (36.9%) were treated with only glucocorticoids and/or csDMARDs (Group A), while eighty-seven patients (63.1%) were treated with biologic drugs (Group B) (Table I). Of note, there were no patients who were treated with only NSAIDs.
| CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IL, interleukin; IVIG, intravenous immunoglobulin; MAS, macrophage activation syndrome; NSAIDs, nonsteroidal anti-inflammatory drugs; sJADAS, systemic Juvenile Arthritis Disease Activity Score; TNF-α, tumor necrosis factor-alpha | ||||
| Table I. Characteristics of systemic juvenile idiopathic arthritis patients treated with glucocorticoids ± conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) (Group A) vs. those treated with biologics (Group B). | ||||
| All patients (n=138) | Group A (n=51) | Group B (n=87) | P value | |
| Age at diagnosis, year, median (Q1-Q3) | 5.5 (1.9-11.6) | 5.7 (2.1-11.8) | 5.2 (1.8-11.4) | 0.114 |
| Sex, female, n (%) | 63 (45.7) | 25 (49.1) | 38 (43.7) | 0.543 |
| Clinical findings at diagnosis, n (%) | ||||
| Fever | 138 (100) | 51 (100) | 87 (100) | - |
| Rash | 82 (59.4) | 30 (58.8) | 52 (59.8) | 0.913 |
| Arthritis | 78 (56.5) | 29 (56.9) | 58 (66.7) | 0.249 |
| Lymphadenopathy | 71 (51.4) | 26 (50.9) | 45 (51.7) | 0.933 |
| Hepatomegaly/splenomegaly | 6 (33.3) | 18 (35.3) | 28 (32.2) | 0.708 |
| Serositis | 15 (10.9) | 5 (9.8) | 10 (11.5) | 0.758 |
| MAS | 53 (38.4) | 12 (23.5) | 45 (51.7) | 0.001 |
| Laboratory findings at diagnosis, median (Q1-Q3) | ||||
| Hemoglobin, gr/dL | 10 (8.9-12.7) | 10.5 (9.2-13) | 9.9 (8.7-12.5) | 0.116 |
| Leukocyte count, x103/mm3 | 14.9 (4.3-24.5) | 15 (4.9-23.9) | 14.6 (3.9-24.8) | 0.208 |
| Platelet count, x103/mm3 | 411 (132-585) | 417 (144-523) | 405 (124-598) | 0.341 |
| CRP, mg/dL (<0.5) | 13.1 (1.5-26.8) | 10.7 (1.1-23.1) | 15.3 (2.1-28.3) | <0.001 |
| ESR, mm/hour (0-20) | 56.7 (33-97) | 55.4 (31-94) | 58.5 (37-101) | 0.056 |
| sJADAS at diagnosis, median (Q1-Q3) | 24.8 (15.1-31.2) | 19.5 (14.3-19.5) | 28.7 (17.9-34.8) | <0.001 |
| Treatment, ever, n (%) | ||||
| NSAIDs | 64 (46.4) | 25 (49.1) | 39 (44.8) | 0.634 |
| Glucocorticoid | 138 (100) | 51 (100) | 87 (100) | - |
| Methotrexate | 48 (34.8) | 17 (33.3) | 31 (35.2) | 0.784 |
| Cyclosporin-A | 17 (12.3) | 6 (11.8) | 11 (12.6) | 0.879 |
| IVIG | 29 (21.1) | 10 (19.6) | 19 (21.8) | 0.756 |
| Biologic drugs | 87 (63.1) | 0 | 87 (100) | - |
| Anakinra | 70 (50.7) | 0 | 70 (80.4) | - |
| Canakinumab | 45 (32.6) | 0 | 45 (51.7) | - |
| Tocilizumab | 29 (21.1) | 0 | 29 (33.3) | - |
| Anti-TNF-α agents | 7 (5.1) | 0 | 7 (8.1) | - |
| Disease course, n (%) | ||||
| Monocyclic | 76 (32.8) | 32 (62.7) | 11 (11.5) | <0.001 |
| Polycyclic | 33 (18.1) | 5 (9.8) | 22 (25.3) | 0.027 |
| Persistent | 74 (40.4) | 14 (27.5) | 38 (43.7) | 0.058 |
| Glucocorticoid withdrawal time, months, median (Q1-Q3) | 5 (1.5-40) | 7 (2-48) | 3 (1-30) | <0.001 |
| Number of patients who discontinued glucocorticoid in the first year, n (%) | 98 (71.1) | 26 (50.9) | 72 (82.8) | <0.001 |
| Transition time to biologic drug, months, median (Q1-Q3) | 3 (3-12) | - | 3 (3-12) | - |
| sJADAS at last visit, median (Q1-Q3) | 0.4 (0-4.6) | 0.4 (0-4.2) | 0.5 (0-4.9) | 0.283 |
| Duration of follow-up, years, median (Q1-Q3) | 6.4 (1.5-8.8) | 6.1 (1.7-8.5) | 6.7 (1.3-9.1) | 0.472 |
| Outcome, n (%) | ||||
| Remission on-drug | 44 (31.9) | 13 (25.5) | 31 (39.1) | 0.217 |
| Remission off-drug | 92 (66.7) | 37 (72.5) | 55 (63.2) | 0.262 |
| Exitus | 2 (1.4) | 1 (1.9) | 1 (1.1) | 1.000 |
Group B patients more frequently had MAS at disease presentation than Group A patients (p=0.001). In addition, acute phase reactant (CRP and ESR) levels and sJADAS at diagnosis in Group B were higher than in Group A (p<0.001 for both). While monocyclic disease course was frequently observed in patients of Group A (p<0.001), polycyclic disease course was more common in patients of Group B (p=0.027). Most of the patients in Group B were able to discontinue glucocorticoids in a shorter period (p<0.001), and the number of patients who discontinued glucocorticoids in the first year was higher (p<0.001).
In the univariate and multivariate logistic regression analyses, history of MAS (OR 3.419, 95% CI 1.194-9.792; p=0.022) and polycyclic disease course (OR 4.351, 95% CI 1.329-14.240; p=0.015), CRP levels of >13.6 mg/dL (OR 2.838, 95% CI 1.182-6.815; p=0.020), and sJADAS levels of >24.1 (OR 4.490, 95% CI 1.725-11.684; p=0.002) at diagnosis were associated with the requirement of biologic drugs in the treatment (Table II).
| CI, confidence interval; CRP, C-reactive protein; MAS, macrophage activation syndrome; sJADAS, systemic Juvenile Arthritis Disease Activity Score | ||
| Table II. Univariate and multivariate regression analysis for predictive factors associated with the requirement of biologic drugs in the treatment of patients with systemic juvenile idiopathic arthritis. | ||
| Variables | OR (%95 CI) | P value |
| Univariate analyses | ||
| MAS at diagnosis | 3.482 (1.610-7.533) | 0.002 |
| Polycyclic disease course | 3.114 (1.099-8.826) | 0.033 |
| CRP at diagnosis >13.6 mg/dL | 2.092 (1.036-4.226) | 0.040 |
| sJADAS at diagnosis >24.1 | 3.760 (1.803-7.838) | <0.001 |
| Multivariate analyses | ||
| MAS at diagnosis | 3.419 (1.194-9.792) | 0.022 |
| Polycyclic disease course | 4.351 (1.329-14.240) | 0.015 |
| CRP at diagnosis >13.6 mg/dL | 2.838 (1.182-6.815) | 0.020 |
| sJADAS at diagnosis >24.1 | 4.490 (1.725-11.684) | 0.002 |
Discussion
In our study, prominent articular symptoms, MAS, and severe/resistant systemic symptoms were major indications for using csDMARDs or biologics in SJIA treatment. History of MAS and polycyclic disease course, CRP >13.6 mg/dL, and sJADAS >24.1 at diagnosis were independent predictors of the requirement for biologic drugs in treatment. Also, in most patients receiving biologic treatment, glucocorticoids were discontinued in a shorter period, and a higher percentage of patients discontinued glucocorticoids within the first year.
In the initial treatment of sJIA, glucocorticoids serve as the first step.13 However, there is currently no consensus regarding the appropriate dosage and duration of steroid therapy. Before biologic drugs were available, sJIA patients were mainly treated with glucocorticoids and csDMARDs.5,6 IL-1 inhibitors (anakinra, canakinumab) and IL-6 inhibitors (tocilizumab) are significantly effective in patients with sJIA.14,15 In the most recent ACR guideline, it is recommended to give anti-IL-1 to patients with predominant systemic findings, and tocilizumab to patients with severe arthritis.6 Even in the biologic treatment era, some sJIA patients are still treated with glucocorticoids alone or in combination with csDMARDs. Glucocorticoids alone are generally preferred in patients with monophasic disease, and the treatment can be discontinued in 3-6 months.7,16 In patients with severe arthritis, MTX is usually added to glucocorticoids, and in those presenting with MAS, Cyc-A is often added.17,18 If there is no response to these treatments during follow-up, it is recommended to switch to biologic treatments.6 In our study, all patients with sJIA were initially given glucocorticoids. Of patients, 20.3% achieved remission with glucocorticoid therapy alone. csDMARDs were started after glucocorticoids in 47.1% of the patients, and biologic drugs were started in 32.6%. While csDMARDs were mostly started for reasons such as significant joint symptoms, MAS at diagnosis, and systemic symptoms that could not be controlled with glucocorticoids, IL-1 inhibitors were also given to patients with severe systemic symptoms and those presenting with MAS. During follow-up, some of the patients (64.6%) using csDMARDs were switched to biologic treatment due to a persistent polyarticular unresponsive course to csDMARDs or resistant/recurrent systemic inflammation.
Adiguzel Dundar et al.7 observed 58 disease episodes in 50 sJIA patients. Forty-one (70.6%) of these episodes were controlled with MTX, following the discontinuation of glucocorticoids. However, a biologic drug was needed in the remaining 17 (29.4%) episodes. Patients receiving MTX were stratified into two groups: Group I (n=36) comprising patients treated with MTX alone, and Group II (n=14) consisting of patients treated with MTX in combination with a biologic agent; Group I patients had mainly a monocyclic disease course (56.1%), while Group II exhibited a higher prevalence of a persistent course (70.6%). Notably, the initial ESR and the neutrophil/lymphocyte ratio (NLR) were found to be significantly elevated in Group II than in Group I (p=0.003 and p=0.007, respectively).7 Similar to this study, there was a significant elevation in acute phase reactants at the time of diagnosis in our patients treated with biologic drugs, and a monocyclic disease course was frequently detected in patients treated with glucocorticoids ± csDMARDs, in our study. However, arthritis and MAS were more common at diagnosis and there were high sJADAS values in the patients treated with glucocorticoids ± csDMARDs. In addition, polycyclic course was more common in our patients in this group.
Biologic agents have demonstrated remarkable efficacy in the treatment of sJIA, effectively reducing the need for glucocorticoids and their associated adverse events.19 Consistent with these, the patients treated with biologics discontinued glucocorticoids in a shorter period in our study. Aydın et al.20 evaluated the treatments and outcomes of 36 sJIA patients in 2020. All patients had received glucocorticoids. Twenty-six (72.2%) were treated with biologics. They reported that the duration of glucocorticoid exposure was significantly reduced after the use of biologic agents (p=0.001).
Finally, in our study, we revealed the necessity of using biologic drugs in initial or follow-up treatments in patients with a history of MAS and polycyclic disease course, CRP >13.6 mg/dL, or sJADAS >24.1 at diagnosis. As far as we know, there has been no study before on this subject in the literature. It was not surprising that these patients had a history of MAS at diagnosis and polycyclic disease course. However, we recommend closer observation and follow-up, especially in patients with significant CRP elevations at diagnosis and high sJADAS scores, because in sJIA, as in many diseases, early treatment is very important to prevent serious morbidity and mortality. It would be beneficial in sJIA patients with these characteristics not to wait too long before initiating biologic treatment.
The primary limitation of this study was its retrospective nature, which makes it vulnerable to potential inaccuracies and erroneous assumptions due to incomplete or incorrect medical records. Another important limitation is the lack of randomization in this study. Future randomized controlled trials will make the results clearer. In addition, the study was a single-center study, which may limit the generalizability of its findings. Finally, we revealed our clinical experiences in our study, but it should not be forgotten that the treatment approach is heterogeneous and individual.
Conclusion
In conclusion, sJIA patients with a history of MAS, polycyclic disease course, significantly high CRP levels and sJADAS values at the time of diagnosis are probably more likely to require biologic drugs in the treatment. In addition, biologic drugs protect patients from long-term glucocorticoid exposure. However, it is also important to point out that sJIA is a complex and variable disease, and the treatment approach needs to be adjusted over time based on the patient’s response and the course of the disease.
Ethical approval
The study was approved by Hacettepe University Ethics Committee (date: 15.06.2021, number: 2021-12). Informed consent was obtained from all parents/patients before inclusion in the study.
Source of funding
The authors declare the study received no funding.
Conflict of interest
Ezgi Deniz Batu received payment for speakers’ bureaus from Novartis. Seza Özen received consultancy fees and payment for speakers bureaus from Novartis and Sobi. Other authors did not declare any conflicts of interest.
References
- Grevich S, Shenoi S. Update on the management of systemic juvenile idiopathic arthritis and role of IL-1 and IL-6 inhibition. Adolesc Health Med Ther 2017; 8: 125-135. https://doi.org/10.2147/AHMT.S109495
- Correll CK, Binstadt BA. Advances in the pathogenesis and treatment of systemic juvenile idiopathic arthritis. Pediatr Res 2014; 75: 176-183. https://doi.org/10.1038/pr.2013.187
- Beukelman T. Treatment advances in systemic juvenile idiopathic arthritis. F1000Prime Rep 2014; 6: 21. https://doi.org/10.12703/P6-21
- Toplak N, Blazina Š, Avčin T. The role of IL-1 inhibition in systemic juvenile idiopathic arthritis: current status and future perspectives. Drug Des Devel Ther 2018; 12: 1633-1643. https://doi.org/10.2147/DDDT.S114532
- DeWitt EM, Kimura Y, Beukelman T, et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2012; 64: 1001-1010. https://doi.org/10.1002/acr.21625
- Onel KB, Horton DB, Lovell DJ, et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Rheumatol 2022; 74: 553-569. https://doi.org/10.1002/art.42037
- Adiguzel Dundar H, Acari C, Turkucar S, Unsal E. Treatment of systemic JIA: when do we need a biologic? Real world data of a single center. Mod Rheumatol 2021; 31: 684-690. https://doi.org/10.1080/14397595.2020.1761079
- Swart JF, de Roock S, Prakken BJ. Understanding inflammation in juvenile idiopathic arthritis: how immune biomarkers guide clinical strategies in the systemic onset subtype. Eur J Immunol 2016; 46: 2068-2077. https://doi.org/10.1002/eji.201546092
- Petty RE, Southwood TR, Manners P, et al. International League of Associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31: 390-392.
- Tibaldi J, Pistorio A, Aldera E, et al. Development and initial validation of a composite disease activity score for systemic juvenile idiopathic arthritis. Rheumatology (Oxford) 2020; 59: 3505-3514. https://doi.org/10.1093/rheumatology/keaa240
- Schneider R, Laxer RM. Systemic onset juvenile rheumatoid arthritis. Baillieres Clin Rheumatol 1998; 12: 245-271. https://doi.org/10.1016/s0950-3579(98)80018-6
- Wallace CA, Ruperto N, Giannini E; Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology International Trials Organization; Pediatric Rheumatology Collaborative Study Group. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004; 31: 2290-2294.
- Schiappapietra B, Varnier G, Rosina S, Consolaro A, Martini A, Ravelli A. Glucocorticoids in juvenile idiopathic arthritis. Neuroimmunomodulation 2015; 22: 112-118. https://doi.org/10.1159/000362732
- Akioka S. Interleukin-6 in juvenile idiopathic arthritis. Mod Rheumatol 2019; 29: 275-286. https://doi.org/10.1080/14397595.2019.1574697
- Ter Haar NM, van Dijkhuizen EP, Swart JF, et al. Treatment to target using recombinant interleukin-1 receptor antagonist as first-line monotherapy in new-onset systemic juvenile idiopathic arthritis: results from a five-year follow-up study. Arthritis Rheumatol 2019; 71: 1163-1173. https://doi.org/10.1002/art.40865
- Vannucci G, Cantarini L, Giani T, et al. Glucocorticoids in the management of systemic juvenile idiopathic arthritis. Paediatr Drugs 2013; 15: 343-349. https://doi.org/10.1007/s40272-013-0038-0
- Boom V, Anton J, Lahdenne P, et al. Evidence-based diagnosis and treatment of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2015; 13: 55. https://doi.org/10.1186/s12969-015-0055-3
- Woo P, Southwood TR, Prieur AM, et al. Randomized, placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum 2000; 43: 1849-1857. https://doi.org/fmpxzp
- Tarp S, Amarilyo G, Foeldvari I, et al. Efficacy and safety of biological agents for systemic juvenile idiopathic arthritis: a systematic review and meta-analysis of randomized trials. Rheumatology (Oxford) 2016; 55: 669-679. https://doi.org/10.1093/rheumatology/kev382
- Aydın F, Kurt T, Tekgöz N, et al. What has changed over the last decade in systemic juvenile idiopathic arthritis? Turkish Journal of Pediatric Disease 2021; 15: 65-71. https://doi.org/10.12956/tchd.807572
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















