Abstract
Background. Congenital lung malformations (CLMs) refer to structural abnormalities of the lungs that occur during fetal development. Matrix metalloproteinases (MMPs) constitute a group of zinc-dependent enzymes, with certain members of this family playing pivotal roles in the remodeling of the lungs both prenatally and postnatally. This study aimed to explore expression levels of MMP-2, MMP-7, and MMP-9 in CLMs which are recognized as pivotal contributors to their clinical pathology.
Methods. A total of 41 patients between the ages of 0-17 years that had undergone lung surgery for CLMs between March 2007- July 2023 were analyzed. The demographic features, clinical and pathological findings were recorded. The expression levels of MMP-2, MMP-7 and MMP-9 in patients’ tissues were examined by reverse transcription polymerase chain reaction and compared in CLMs and adjacent normal lung tissues.
Results. Among patients with CLMs, 12 patients had congenital pulmonary airway malformations (CPAM, one patient had bilateral lesions), 18 patients had bronchopulmonary sequestration (BPS), 7 patients had congenital lobar overinflation (CLO), and 4 patients had bronchogenic cyst (BC). The higher expression of MMP-7 and MMP-9 in all CLM tissues compared to normal tissue was observed. But, there was a trend in MMP-2 expression in CPAM tissues and MMP-2 showed high expression in the BPS, CLO and BC groups, which was not statistically significant. Upon collective analysis of all groups, it was observed that mRNA expressions of MMP-7 and MMP-9 exhibited greater upregulation in CPAM and BC in comparison to BPS and CLO.
Conclusions. Our findings indicate a specific involvement of MMP-7 and MMP-9 in the pathogenesis of CLMs, particularly in CPAM and BC. To the best of our knowledge, this research represents the initial demonstration of MMP expression in CLMs.
Keywords: bronchogenic cyst, congenital lung malformations, congenital pulmonary airway malformations, Matrix metalloproteinase-7 (MMP-7), Matrix metalloproteinase-9 (MMP-9)
Introduction
Congenital lung malformations (CLMs) have an estimated prevalence of 3.5 per 10.000 births.1 However, their apparent incidence is increasing with recent data suggesting that CLMs may be identified prenatally in approximately 1 in 2.400 live births.2 Congenital lung lesions encompass a wide-ranging group of disorders, the most common of which include congenital pulmonary airway malformation (CPAM, previously known as congenital cystic adenomatoid malformation), bronchopulmonary sequestration (BPS), congenital lobar overinflation (CLO; previously known as congenital lobar emphysema), and bronchogenic cyst (BC).
The pathogenesis of congenital lung lesions is not yet fully known. CLMs are understood as resulting from a focal development malformation and present as cystic lesions.3 The hypothesis proposed by Langston suggests that CLMs can be attributed to variations in airway obstruction in utero and that the level, timing, and completeness of the obstruction produce different patterns of malformation.4,5 Recent studies have shown that bronchial atresia/obstruction is a possible hidden pathology underlying many congenital lung lesions, leading to cystic maldevelopment.6-8 This focal airway obstruction leads to focal hypoxia, inflammation, and tissue damage. Matrix metalloproteinases (MMPs) comprise a family of zinc-dependent enzymes and several members of the matrix metalloproteinase family are critical in lung remodeling before and after birth.9 Overexpression of MMPs is shown in inflammatory conditions.10 Therefore, we hypothesize that overexpression of MMPs in CLMs indicates that CLM may result from tissue repair and remodeling induced by inflammation related to intrauterine airway obstruction. To our knowledge, this is the first study to focus on this subject.
The aim of this study was to investigate the expression of MMP-2, MMP-7 and MMP-9 which have been marked as being critical for the clinical pathology of lung diseases.
Materials and Methods
Clinical characteristics of the patients and study design
Medical records and tissue specimens of 55 patients that had undergone lung surgery for CLMs at the Department of Pediatric Surgery of Bursa Uludag University Hospital between March 2007- July 2023 were retrospectively analyzed. The demographic features, prenatal diagnosis history, clinical and pathological findings were recorded. Cases were considered to be symptomatic if they had acute respiratory distress, pneumothorax, recurrent pneumonia, cough, and inability to feed. Asymptomatic cases were those who did not exhibit these symptoms. In our clinic, we perform surgery in symptomatic cases, even during the neonatal period. In asymptomatic cases, we perform elective surgery after the postnatal 3rd month. We prefer segmentectomy or lobectomy techniques by thoracotomy or thoracoscopy according the computed tomography images.
Fourteen patients were excluded from the study due to lack of data and signs of infection or presence of inflammatory cells in the tissue specimens. A total of 41 patients between the ages of 0-17 years were included in the study. Institutional ethics approval was provided for the study.
RNA isolation and quantitative reverse transcriptase polymerase chain reaction
(qRT-PCR)
Formalin-fixed paraffin-embedded (FFPE) cores containing congenital lung malformations were marked and total RNA was isolated from each. Total RNA was isolated from CLM tissue and normal lung tissues using the RNeasy FFPE Kit (Qiagen, USA) as per the manufacturer’s protocol. The RNA quality (A260/A280 ratios) and quantity (ng/μl) were assessed spectrophotometrically (Beckman Coulter), and samples were stored at -80°C. For cDNA synthesis, 300 ng of RNA was used with the High Capacity cDNA Reverse Transcription Kit protocol (Applied Biosystems), and the resulting cDNA samples were stored at -20°C. Gene expression analyses for MMP-2, MMP-7, and MMP-9 were conducted using the ABI StepOnePlus™ Real-Time PCR System (Applied Biosystems). Each 20-μl reaction contained cDNA, TaqMan™ Gene Expression Master Mix, and TaqMan™ Gene Expression Assays, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as the endogenous control. The amplification process included a melting curve analysis to ensure the specificity of the reactions, and cycle threshold (Ct) values were recorded. Gene expression differences were quantified using the Comparative Cycle Threshold method. According to this method, gene expression levels were calculated using the ΔCt (Delta Ct) method, where the Ct value of the target gene was normalized to that of the reference gene. The relative gene expression differences between groups were determined using the ΔΔCt (Delta Delta Ct) method and expressed as fold change using the formula 2−ΔΔCt.
Statistical analysis
All statistical analyses were performed using SPSS statistical software for Windows, version 28.0 (SPSS, Chicago, IL, USA). Two-tailed Student’s t-test was used to compare the MMP-2, MMP-7, and MMP-9 levels between CLMs and adjacent normal lung tissues. All data were presented as the mean ± standard deviation, where p<0.05 was considered statistically significant.
Results
A total 41 patients with CLMs were included in the study. There were 13 lesions of CPAM (one patient had bilateral lung lesions), 18 lesions of BPS, 7 lesions of CLO, and 4 lesions of BC. Table I describes patient demographic characteristics and clinical features, including the age at the time of operation and operative techniques used. All patients underwent thoracotomy except 3 patients. Three patients with BPS underwent thoracoscopic surgery. The comparison of MMP mRNA levels across age groups in CPAM, BPS, CLO and BC was currently statistically nonsignificant due to low number of patients. A total of 18 patients had symptoms in all groups. No statistical significance was determined between MMPs and symptoms (p>0.05).
|
* In one patient with bilateral CPAM, right lobectomy and left segmentectomy were performed at different times. The same patient is shown separately as lobectomy and segmentectomy in the table. **Only lesion excision: 9 for BPS, 4 for BC. The patients with BPS had 8 of extralobar and 10 of intralobar type. ***Classification for CPAM is Stocker’s classification. 1 patient with bilateral CPAM is shown separately as type 1 and type 2 in the table, due to having different types. Pathological typing was not performed in 1 patient. **** Hybrid type refers to the lung lesion comprising both BPS and CPAM type 2. BC: Bronchogenic cyst, BPS: Bronchopulmonary sequestration, CLO: Congenital lobar overinflation, CPAM: Congenital pulmonary airway malformation. |
||||
| Table I. Demographic characteristics, clinical features and operative techniques (N:41). | ||||
| Patients (n) | CPAM (n:12) | BPS (n:18) | CLO (n:7) | BC (n:4) |
| Prenatal diagnosis | 8 | 15 | 3 | 0 |
| Age at operation time Median (Min-Max) |
240 days (5-2190) |
135 days (4-1095) |
4.5 months (1-12) |
24 months (1-204) |
| Gender (Female/Male) | 1/1 | 1/2 | 2/5 | 1/1 |
|
Symptomatic (n) Asymptomatic (n) |
5 7 |
5 13 |
5 2 |
3 1 |
| Operative technique-1 | ||||
| Thoracotomy (n) | 12 | 15 | 7 | 4 |
| Thoracoscopy (n) | 0 | 3 | 0 | 0 |
| Operative technique-2 | ||||
| Segmentectomy (n) | 9* | 4** | 0 | 0** |
| Lobectomy (n) | 4* | 5** | 7 | 0** |
| Histopathological type (n) |
Stocker’s classification*** Type 0: 0 Type 1: 7 Type 2: 5 Type 3: 0 Type 4: 0 |
Hybrid type****: 3 | - | - |
The expression profiles of MMP-2, MMP-7, and MMP-9 in the lesion compared with adjacent normal tissues by comparative Ct method (2−ΔΔCt) are shown in Table II.
| BC: Bronchogenic cyst, BPS: Bronchopulmonary sequestration, CLO: Congenital lobar overinflation, CPAM: Congenital pulmonary airway malformation, MMP: Matrix metalloproteinase. | |||
| Table II. The expression profiles of MMP-2, MMP-7, and MMP-9 in the lesion compared with adjacent normal tissues by comparative Ct method (2−ΔΔCt). | |||
|
|
2−ΔΔCt values | ||
| MMP-2 | MMP-7 | MMP-9 | |
| CPAM | 0.673 | 4.103 | 3.187 |
| CLO | 0.745 | 1.145 | 1.456 |
| BPS | 0.678 | 1.245 | 1.678 |
| BC | 0.567 | 1.993 | 3.373 |
MMP-7 and MMP-9 mRNA expressions were significantly higher in CPAM tissues as compared to that of adjoining normal lung tissues (MMP-7; 2-ΔΔCT: 4.103 ± 0.319, p<0.0001, MMP-9; 2-ΔΔCT: 3.187 ± 0.641, p<0.001). There was a trend in MMP-2 mRNA expression in CPAM tissues compared to normal tissue (MMP-2; 2-ΔΔCT: 0.673 ± 0.164, p=0.06).
BC tissues also had significantly higher expression of MMP-7 and MMP-9 mRNA compared to the normal lung tissues (MMP-7; 2-ΔΔCT: 1.993 ± 0.149, p<0.05, MMP-9; 2-ΔΔCT: 3.373 ± 0.153, p<0.001). Although MMP-2 showed high expression in the BC group, it was not statistically significant (MMP-2; 2-ΔΔCT: 0.567 ± 0.0605, p=0.10).
MMP-2, MMP-7 and MMP-9 expressions were higher in tissues of patients diagnosed with BPS and CLO compared to the normal lung, but this difference was not statistically significant.
When all groups were evaluated together, MMP-7 and MMP-9 mRNA expressions showed higher increases in CPAM and BC compared to BPS and CLO (Fig. 1 and Fig. 2).
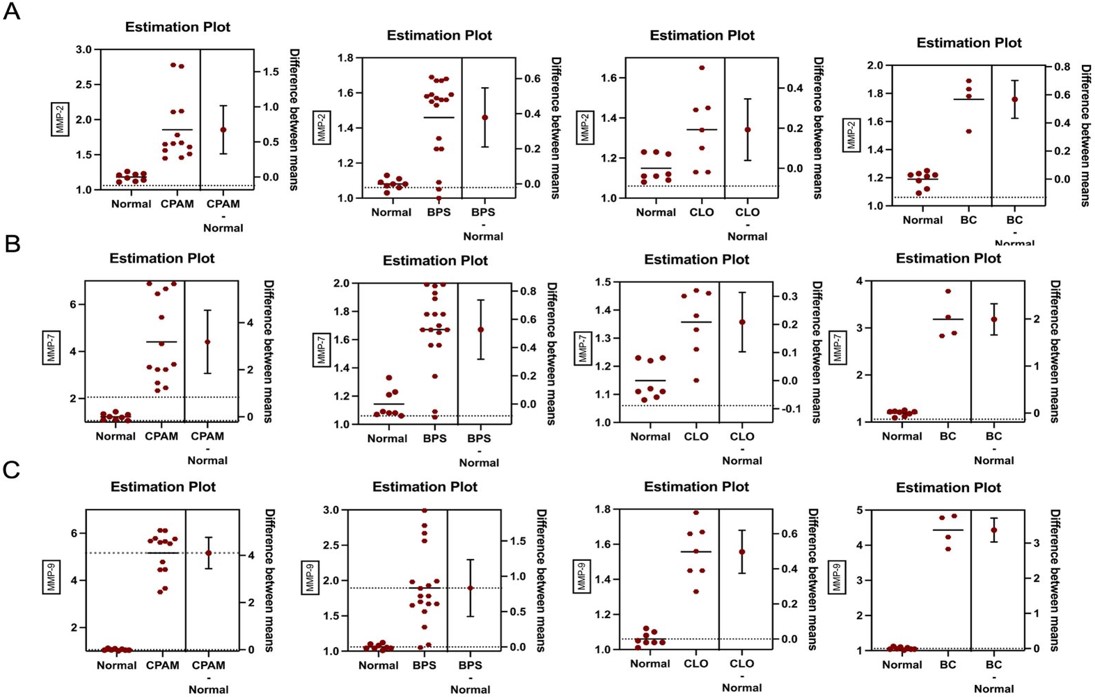
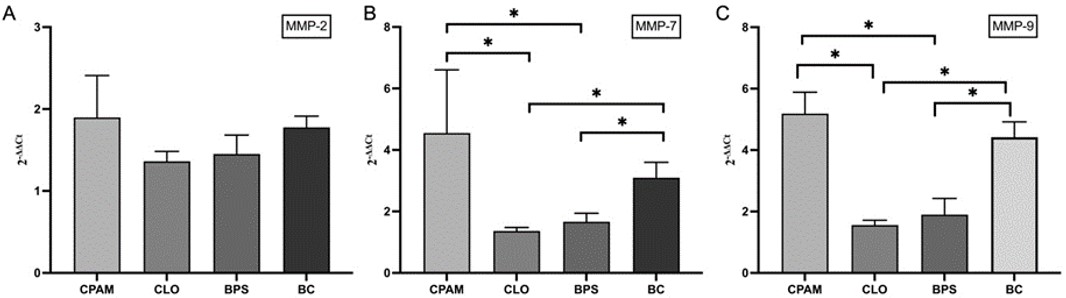
BC: Bronchogenic cyst, BPS: Bronchopulmonary sequestration, CLO: Congenital lobar overinflation, CPAM: Congenital pulmonary airway malformation, MMP: Matrix metalloproteinase.
Discussion
MMPs are a family of zinc-dependent endopeptidases. They are involved in a number of normal and pathological processes. Degradation of the extracellular matrix was regarded the primary function for MMPs, but several other functions have also been associated with MMPs, including signaling for cell growth, inflammation and angiogenesis.11 MMP-2 and MMP-14 are constitutively expressed throughout lung development, and their expression diminishes once lung development is completed. These may become upregulated again in response to disease states later in life. Other MMPs ( MMP-12, -3, -9, -7, and -21 ) are not expressed early in lung development, but their expression is induced in response to injury or environmental causes.9 MMP-2 and MMP-9 are particularly important in the pathogenesis of inflammatory, infectious and neoplastic diseases in many organs including the lung.10,12 MMP-7 is another subgroup of MMPs, maintaining innate immunity in lungs and is overexpressed in malignant transformations.13 Here, we investigated the expression levels of MMP-2, MMP-7, and MMP-9 in healthy lungs and CLMs lesion.
The majority of MMPs are not expressed in normal healthy tissues, but are expressed in diseased tissues that are inflamed or undergoing repair and remodeling.14 Pelizzo et al.15 reported a 79% incidence of pulmonary inflammation in patients with CPAM, who underwent surgery during early postnatal period and within 3 months of life, and added that early signs of inflammation can be present even in asymptomatic infants. They questioned whether this inflammatory process begins before birth. They suggested that the presence of inflammation without signs of infections suggests a form of inflammatory reaction induced by the malformation itself. In fact, in our study, MMPs which are known to increase during inflammation were overexpressed in CPAMs and other types of CLMs, although we excluded CLM samples with any sign of infection or presence of inflammatory cells in tissue samples. Furthermore, in our study, no statistically significant relationship was found between MMPs and symptoms (p>0.05). The reasons that initiate this inflammatory process are still unclear. Focal airway obstruction may initiate the inflammatory process.
CLMs are a heterogeneous group but hybrid lesion or occasional coexistence of these cystic lesions suggest that there may be a single pathologic mechanism for their development.16 Level, completeness, and timing of the in-utero airway obstruction determine the different patterns of lung malformation.4,16 The recent studies have shown that bronchial atresia/obstruction is associated with all types of developmental lung malformations.6-8 Tang et al.17 reported a positive correlation between the levels of MMP-9 and the length of foreign body retention. They suggested that mechanical obstruction of foreign bodies leads to the enhanced generation of MMP-9 and also eventual airway remodeling. The overexpression of MMPs leads to matrix breakdown, tissue destruction, and cystic lesions.18 We found that MMP-7 and MMP-9 mRNA expressions showed higher increases in CPAM, BC, BPS and CLO, which indicated inappropriate remodeling in the lung tissue. The underlying reason is unclear, but a focal obstructive process of the tracheobronchial tree is proposed to explain the pathophysiology.4
The management of a newborn with a symptomatic lesion is surgery, but management of an asymptomatic one can be controversial. The main argument in favor of routine resection during infancy is the long term risk of infection or malignancy.5 All histological types of CLMs may be associated with malignant lung lesions.19,20 Little is known about underlying pathophysiology of CLMs and the processes that may promote their malignant transformation.1 In addition, elevated expression levels of MMPs are associated with several cancers. MMP-2, MMP-7 and MMP-9 have essential roles in tumor angiogenesis and in a broader perspective for tumor development. MMPs have been investigated in lung cancer for prognostic factor or potential therapeutic targets.12,21 In our study, a higher expression of MMP-7 and MMP-9 was found in the all CLM groups but there was a trend in MMP-2 mRNA expression in CPAM tissues compared to normal tissue. MMP-2 showed high expression in the BPS, CLO and BC groups, but was not statistically significant. In their comprehensive review on the association between CLMs and lung tumors in children and adults, Casagrande et al.20 found that the CLM that was most often associated with lung tumor was CPAM, followed by BC. They also mentioned that malignant malformation of CLMs might be secondary to prolonged inflammatory episodes and metaplastic changes as chronic inflammatory diseases have been recognized to stimulate neuroendocrine cell proliferation. In our study, when all groups were evaluated together, MMP-7 and MMP-9 mRNA expressions showed higher increases in CPAM and BC compared to BPS and CLO. While our findings suggest that an association can be established between congenital lung lesion, inflammation and tumor development, more evidence is needed to explain this association.
Our study highlights the significant overexpression of MMP-7 and MMP-9 in congenital lung malformations, particularly in CPAM and BC, compared to BPS and CLO, suggesting a potential link between inflammation, extracellular matrix remodeling and tumorigenesis. The elevated levels of these MMPs underscore their role in the inappropriate remodeling of lung tissue and raise concerns regarding their contribution to the malignant transformation of CLMs. Although MMP-2 expression showed an upward trend in CPAM, BPS, CLO, and BC, it was not statistically significant.
The results reinforce the hypothesis that inflammation, possibly triggered by focal airway obstruction or intrinsic malformation, could be a driving factor in the pathophysiology of CLMs. This aligns with prior research suggesting that prolonged inflammatory episodes and matrix breakdown may facilitate tumorigenesis.
Considering the elevated expression of MMP-7 and MMP-9 in CLMs and their established role in tumor development and angiogenesis, our findings provide further support for the potential utility of MMPs as biomarkers or therapeutic targets in lung disease. However, more extensive studies are needed to establish the precise mechanisms linking CLMs, inflammation, and tumor development, as well as to clarify the role of MMP-2 in this process.
Ethical approval
The study was approved by the Bursa Uludağ University Ethics Committee Institutional ethical committee approval was provided for the study (Date: 06.03.2024 Approval no: 2024-3/2).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Doktor F, Antounians L, Lacher M, Zani A. Congenital lung malformations: dysregulated lung developmental processes and altered signaling pathways. Semin Pediatr Surg 2022; 31: 151228. https://doi.org/10.1016/j.sempedsurg.2022.151228
- Stocker LJ, Wellesley DG, Stanton MP, Parasuraman R, Howe DT. The increasing incidence of foetal echogenic congenital lung malformations: an observational study. Prenat Diagn 2015; 35: 148-153. https://doi.org/10.1002/pd.4507
- Correia-Pinto J, Gonzaga S, Huang Y, Rottier R. Congenital lung lesions-underlying molecular mechanisms. Semin Pediatr Surg 2010; 19: 171-179. https://doi.org/10.1053/j.sempedsurg.2010.03.003
- Langston C. New concepts in the pathology of congenital lung malformations. Semin Pediatr Surg 2003; 12: 17-37. https://doi.org/10.1053/spsu.2003.00001
- Singh R, Davenport M. The argument for operative approach to asymptomatic lung lesions. Semin Pediatr Surg 2015; 24: 187-195. https://doi.org/10.1053/j.sempedsurg.2015.02.003
- Kunisaki SM, Fauza DO, Nemes LP, et al. Bronchial atresia: the hidden pathology within a spectrum of prenatally diagnosed lung masses. J Pediatr Surg 2006; 41: 61-65. https://doi.org/10.1016/j.jpedsurg.2005.10.082
- Riedlinger WF, Vargas SO, Jennings RW, et al. Bronchial atresia is common to extralobar sequestration, intralobar sequestration, congenital cystic adenomatoid malformation, and lobar emphysema. Pediatr Dev Pathol 2006; 9: 361-373. https://doi.org/10.2350/06-01-0023.1
- Fowler DJ, Gould SJ. The pathology of congenital lung lesions. Semin Pediatr Surg 2015; 24: 176-182. https://doi.org/10.1053/j.sempedsurg.2015.02.002
- Hendrix AY, Kheradmand F. The role of matrix metalloproteinases in development, repair, and destruction of the lungs. Prog Mol Biol Transl Sci 2017; 148: 1-29. https://doi.org/10.1016/bs.pmbts.2017.04.004
- Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res 2005; 31: 599-621. https://doi.org/10.1080/019021490944232
- Stenvold H, Donnem T, Andersen S, et al. Overexpression of matrix metalloproteinase-7 and -9 in NSCLC tumor and stromal cells: correlation with a favorable clinical outcome. Lung Cancer 2012; 75: 235-241. https://doi.org/10.1016/j.lungcan.2011.06.010
- Christopoulou ME, Papakonstantinou E, Stolz D. Matrix metalloproteinases in chronic obstructive pulmonary disease. Int J Mol Sci 2023; 24: 3786. https://doi.org/10.3390/ijms24043786
- Soyer T, Birben E, Akıncı SM, et al. The miRNA-24, miRNA-21 expressions and matrix metalloproteinase-7 level in exhaled breath condensate of children with primary spontaneous pneumothorax. J Breath Res 2022; 17: 016007. https://doi.org/10.1088/1752-7163/aca928
- Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 2006; 61: 259-266. https://doi.org/10.1136/thx.2005.051979
- Pelizzo G, Barbi E, Codrich D, et al. Chronic inflammation in congenital cystic adenomatoid malformations. An underestimated risk factor? J Pediatr Surg 2009; 44: 616-619. https://doi.org/10.1016/j.jpedsurg.2008.10.064
- Shanti CM, Klein MD. Cystic lung disease. Semin Pediatr Surg 2008; 17: 2-8. https://doi.org/10.1053/j.sempedsurg.2007.10.002
- Tang LF, Du LZ, Chen ZM, Zou CC. Extracellular matrix remodeling in children with airway foreign-body aspiration. Pediatr Pulmonol 2004; 38: 140-145. https://doi.org/10.1002/ppul.20071
- Pimenta SP, Baldi BG, Nascimento EC, Mauad T, Kairalla RA, Carvalho CR. Birt-Hogg-Dubé syndrome: metalloproteinase activity and response to doxycycline. Clinics (Sao Paulo) 2012; 67: 1501-1504. https://doi.org/10.6061/clinics/2012(12)25
- Hall NJ, Stanton MP. Long-term outcomes of congenital lung malformations. Semin Pediatr Surg 2017; 26: 311-316. https://doi.org/10.1053/j.sempedsurg.2017.09.001
- Casagrande A, Pederiva F. Association between congenital lung malformations and lung tumors in children and adults: a systematic review. J Thorac Oncol 2016; 11: 1837-1845. https://doi.org/10.1016/j.jtho.2016.06.023
- Kowalczyk A, Nisiewicz MK, Bamburowicz-Klimkowska M, et al. Effective voltammetric tool for simultaneous detection of MMP-1, MMP-2, and MMP-9; important non-small cell lung cancer biomarkers. Biosens Bioelectron 2023; 229: 115212. https://doi.org/10.1016/j.bios.2023.115212
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















