Abstract
Background. Duchenne muscular dystrophy (DMD) is a progressive X-linked dystrophinopathy with onset in early childhood. Affected individuals present predominantly with proximal lower limb weakness and pseudohypertrophy of calf musculature being a prominent sign, heralding the onset of contractures in the large joints of lower limbs. Kocher-Debre-Semelaigne syndrome (KDSS) refers to the muscular pseudohypertrophy that develops in children with long-standing hypothyroidism.
Case Presentation. We present an 11-year-old boy with progressive walking difficulty for two years and associated decrease in appetite and chronic constipation. Physical examination revealed mild soft goitre, proximal lower limb weakness, areflexia (except for preserved weak ankle reflex), soft hypertrophy of bilateral calf muscles and latissimus dorsi, with bilateral dynamic ankle joint contractures. Investigations showed moderately elevated total serum creatine phosphokinase (CPK) levels, elevated serum thyroid stimulating hormone (TSH), low free T4, normal free T3 and elevated serum anti-thyroid peroxidase and anti-thyroglobulin antibody titers. A diagnosis of hypothyroidism secondary to Hashimoto’s thyroiditis with Kocher-Debre-Semelaigne syndrome (KDSS) (thyroid myopathy) was made while multiplex ligation-dependent probe amplification confirmed DMD. He was started on steroids and levothyroxine. On follow up, he had improvement in activity, appetite and motor movements (North Star Ambulatory Assessment score 3 to 7).
Conclusion. As a very rare coincidence, our patient suffered from two different diseases with similar presentation which are DMD and KDSS. Subtle clinical clues of joint contractures and goitre helped us identify these unrelated co-existing diseases. An alternate diagnosis must be thought of when all clinical findings cannot be explained by a single disease.
Keywords: Duchenne muscular dystrophy, Kocher-Debre-Semelaigne syndrome, dystrophinopathy, hypothyroidism, pseudohypertrophy
Introduction
Duchenne muscular dystrophy (DMD) is a progressive X-linked dystrophinopathy that results mostly from large deletions affecting the dystrophin gene.1 Affected children present predominantly with proximal lower limb weakness and pseudohypertrophy of calf musculature with weakness progressing gradually and leading to contractures in the large joints of lower limbs. Kocher-Debre-Semelaigne syndrome (KDSS) refers to the muscular pseudohypertrophy that develops in children with long-standing hypothyroidism.2 Creatine phosphokinase (CPK) levels are elevated in both conditions, making the differentiation of the two difficult. Both these conditions can lead to worsening of ambulation with cardiac dysfunction being evident in both conditions. Muscle weakness usually improves to normal within a few weeks to months after initiation of levothyroxine therapy in thyroid myopathy. Hence, the course of illness and treatment response is a good clinical predictor for differentiation between the two conditions. DMD has a more severe course with gradual worsening of ambulation despite treatment with steroids. We would like to emphasize the importance of a thorough clinical examination to pick clues towards an alternative diagnosis when all clinical findings cannot be explained by a single disease. It is important not to miss the diagnosis of a treatable condition with a high index of clinical suspicion and easily performed thyroid function tests.
Case presentation
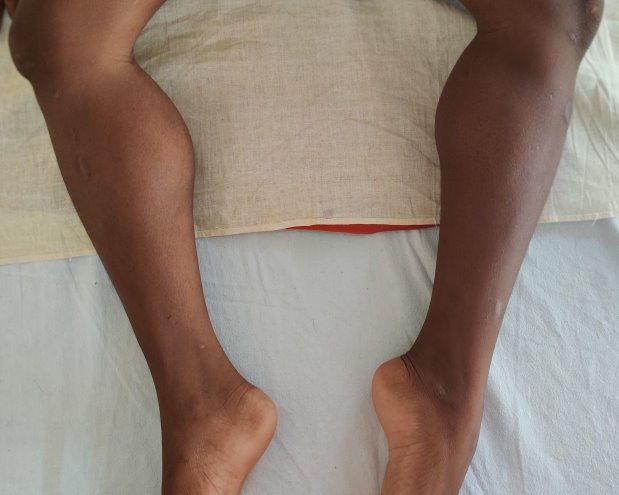
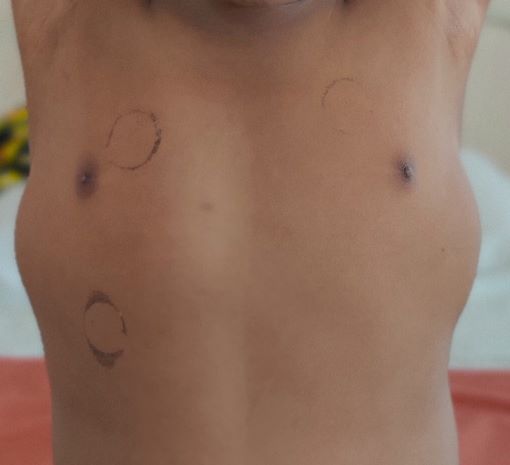
An 11-year-old developmentally normal boy with normal intelligence, firstborn to non-consanguineous parents, presented to us with progressive difficulty in walking for two years that gradually progressed to need for support to stand for the past two months. On probing further, a history of frequent falls while walking for the past 5 years was reported by parents, although no medical support was sought then. There was a history of decreased appetite and chronic constipation for 2 years but no muscle pain, dark colored urine, breathlessness, or cold intolerance. There was no relatable family history. On examination, anthropometric assessment was normal for age (weight 37 kg, height 144 cm, body mass index 17.8 kg/m2). Genital examination revealed Tanner’s stage 2 which was normal for age. A soft swelling of the thyroid gland was noted. Lower limb proximal weakness, areflexia (except for preserved weak ankle reflex), hypertrophy of bilateral calf muscles (soft on palpation) (Fig. 1) and latissimus dorsi (Fig. 2) with bilateral dynamic ankle joint contractures were remarkable. Upper limbs were normal. He had a mild truncal weakness. Gower’s sign could not be elicited as he could not stand without support. North Star Ambulatory Assessment (NSAA) score at admission was 3. Higher mental functions and intellect were normal on examination. The possibility of muscular dystrophy was considered. Total serum CPK levels were moderately elevated (3979 U/L) with elevated aspartate aminotransferase (AST) levels (205 U/L). Thyroid function tests, performed in view of adolescent age for puberty, goitre or autoimmune thyroiditis, revealed elevated serum thyroid stimulating hormone (TSH, 27.8 µIU/L), low free T4 (0.75 ng/dL) and normal free T3 (3.5 pg/mL). Serum anti-thyroid peroxidase (>1300 units/mL) and anti-thyroglobulin (>500 IU/mL) titers were elevated. A diagnosis of hypothyroidism secondary to Hashimoto’s thyroiditis with KDSS (thyroid myopathy) was made. He was started on oral levothyroxine supplementation at 2 µg/kg/day (50 µg per day). Given the progressive joint contractures, dystrophinopathy was considered the most likely cause of muscular dystrophy . Electromyography revealed slow conduction and low amplitude motor units, a myopathic pattern, which could not differentiate between DMD and KDSS. Multiplex ligation-dependent probe amplification (MLPA) was performed for the Dystrophin gene. It revealed deletion of exons 49 and 50, confirming the diagnosis of DMD. He was started on deflazacort orally at 0.9 mg/kg once a day and regular physiotherapy. Echocardiogram revealed mild left ventricular systolic dysfunction (ejection fraction 50% [normal > 55%]), suggestive of early cardiomyopathy. Though asymptomatic at the time, he was started on enalapril as progression over time was expected. The patient was regularly followed for 12 months. Thyroid function tests at 8 months of follow up revealed normal serum TSH (4.1 µIU/L), normal free T4 (1.94 ng/dL) and normal free T3 (3.3 pg/mL). Serum antibody titres (anti-thyroid peroxidase: 699 units/mL and anti-thyroglobulin 254 IU/mL) had decreased and the thyroid swelling had reduced in size on follow up. The child reported improvement in appetite and in the ability to stand and walk with support, with improvement in NSAA score to 7 at the end of follow up.
Informed consent was obtained from the parents for publication of this report. Genetic counselling was provided to the family regarding the management of the patient and the risk of recurrence in blood relatives.
Discussion
DMD, a relentlessly progressive and life-limiting neuromuscular disease is the most common muscular dystrophy (one in 3500 males worldwide).1,3 It is an X-linked dystrophinopathy with males most affected in late childhood. The most common pathology is large deletions in the DMD gene in about 70 % of patients, and point mutations or partial duplications in few.1 Affected individuals usually present by five to seven years of age with predominant proximal lower limb weakness which rapidly progresses to truncal weakness. Pseudohypertrophy of muscles seen in our patient is a prominent sign that progresses from soft and flabby to firm and fibrous over time, heralding the onset of contractures in the large joints of lower limbs. The apparent late onset of symptoms of DMD as in our patient may happen when parents do not notice the mild early symptoms. They usually become wheelchair bound by 11-12 years of age and succumb to cardiac or respiratory illness by late teens.
KDSS, on the other hand, is the name given to muscular pseudohypertrophy that develops in children with long-standing hypothyroidism.2 Its incidence is <10% among patients with thyroid myopathy. It is more common in the age group of 3-10 years. Hashimoto’s thyroiditis diagnosed in our patient, is a common cause and has female preponderance, unlike KDSS.4 Individuals with hypothyroidism may present with lethargy, somnolence, cold intolerance and sometimes depression, or may not have other symptoms of hypothyroidism, making the diagnosis difficult. They can also have symptoms due to muscular involvement like easy fatigability, cramps and stiffness which has been collectively called thyroid myopathy.5
Both DMD and hypothyroidism can affect the functioning of the heart. Patients with DMD may develop cardiomyopathy usually by the second decade of life, however, they often are relatively asymptomatic due to limited activity and mobility with disease progression.6 Hypothyroidism is also associated with cardiac problems, bradycardia, pericardial effusion, reduced cardiac output, reduced ejection fraction (as seen in our patient) and cardiac failure if untreated.7 Thus, a reduced functioning of the heart does not help to differentiate the two conditions.
CPK levels are usually significantly elevated in DMD. Moderate elevation can be seen later in the course of the disease due to significant muscle fibrosis, as in our patient. In a previous study, CPK was shown to have a sensitivity and negative predictive value of 100% and specificity and positive predictive values of 91% and 88.8% respectively in DMD.8
The CPK levels are also elevated in patients with hypothyroidism, including KDSS, again making it difficult to differentiate the two conditions. Skeletal muscles have type 2 deiodinase enzyme which converts T4 to T3 required for muscle function. Hypothyroidism-induced impaired metabolism causes the accumulation of glycogen, glycosaminoglycans and connective tissue, leading to muscular hypertrophy.9 Pseudohypertrophy usually occurs in the limbs, tongue and facial muscles and can bring in a diagnostic conundrum as in our case.
According to the American Thyroid Association guidelines, an increased serum CPK or serum lactate dehydrogenase (LDH) persisting for more than 2 weeks, even if asymptomatic, warrants a TSH level to be obtained to rule out thyroid myopathy.10 Muscle weakness usually improves to normal within a few weeks to months after the initiation of levothyroxine therapy.2,9 Hence, the best clinical predictor to differentiate between DMD and KDSS is the course of disease and response to treatment.
Our patient represents a rare instance of two distinct conditions with overlapping presentations, DMD and KDSS. Distinction of certain characteristics between the two conditions can aid in establishing a diagnosis (Table I). Both of these conditions can lead to worsening of ambulation. The presence of joint contractures and a just perceptible thyroid gland swelling were the clinical clues that led to a diagnosis of both conditions. DMD has a more severe course with gradual worsening of ambulation despite treatment with steroids. The presenting features of our patient posed a diagnostic challenge, raising the question of whether we were faced with a case of DMD with Hashimoto’s thyroiditis or DMD with KDSS. The improvement in our patient’s NSAA score from 3 to 7 over 12 months could be due to a combination of steroid therapy, physiotherapy, and appropriate management of the co-existing hypothyroidism. Although the dilemma still prevails, this response represents a more significant improvement than typically expected solely from steroid therapy in DMD, suggesting a multifactorial contribution, including the resolution of hypothyroid-related symptoms. A high index of suspicion and easily performed thyroid function tests help in avoidance of labelling this highly treatable condition as DMD, which carries a poor prognosis until definitive treatments become accessible and affordable. It is important not to miss the diagnosis of a treatable condition that can significantly improve the quality of life of the patient.
| Table I. Features common to and distinguishing between DMD and KDSS. | ||
| Features common to DMD and KDSS | ||
|
Fatigue and weakness Calf muscle pseudohypertrophy Proximal muscle weakness Elevated CPK levels Hyporeflexia/areflexia Non specific myopathic pattern on EMG |
||
| Features distinct between DMD and KDSS | ||
| DMD | KDSS | |
| Progression of symptoms | Progressive difficulty in walking with history of frequent falls points more towards DMD. | Weakness in KDSS is symmetrical between limbs and is usually not progressive, and shows response to treatment in weeks to months |
| Contractures | More common with DMD | Less likely due to KDSS but can still occur with prolonged muscle weakness. |
| Truncal weakness | Common in DMD | Not a typical feature of KDSS |
| Reflexes | Hyporeflexia/ areflexia | Delayed relaxation of reflexes seen |
| CPK levels | Elevated significantly | Moderate elevation |
| Cardiac findings | Cardiomyopathy more common in DMD | Unusual in KDSS |
| Genetic testing | Dystrophin gene deletion | No genetic predilection |
Thyroid function tests should be performed in all children with muscle hypertrophy and weakness to diagnose KDSS since it is a treatable cause with a good prognosis. DMD and KDSS can have similar clinical presentations and can rarely co-exist. An alternate diagnosis should be looked for when all clinical findings cannot be explained by a single disease.
Ethical approval
Written informed consent for publication of the child’s clinical details was obtained from the concerned parents.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Nassoro DD, Torres L, Marando R, Mboma L, Mushi S, Habakkuk Mwakyula I. A child with duchenne muscular dystrophy: A case report of a rare diagnosis among Africans. Clin Case Rep 2020; 8: 2654-2660. https://doi.org/10.1002/ccr3.3254
- Shaw C, Shaw P. Kocher-debre-semelaigne syndrome: hypothyroid muscular pseudohypertrophy-a rare report of two cases. Case Rep Endocrinol 2012; 2012: 153143. https://doi.org/10.1155/2012/153143
- Sinha R, Sarkar S, Khaitan T, Dutta S. Duchenne muscular dystrophy: Case report and review. J Family Med Prim Care 2017; 6: 654-656. https://doi.org/10.4103/2249-4863.222015
- Ralli M, Angeletti D, Fiore M, et al. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev 2020; 19: 102649. https://doi.org/10.1016/j.autrev.2020.102649
- Mastaglia FL, Ojeda VJ, Sarnat HB, Kakulas BA. Myopathies associated with hypothyroidism: a review based upon 13 cases. Aust N Z J Med 1988; 18: 799-806. https://doi.org/10.1111/j.1445-5994.1988.tb00185.x
- Adorisio R, Mencarelli E, Cantarutti N, et al. Duchenne Dilated Cardiomyopathy: Cardiac Management from Prevention to Advanced Cardiovascular Therapies. J Clin Med 2020; 9: 3186. https://doi.org/10.3390/jcm9103186
- Udovcic M, Pena RH, Patham B, Tabatabai L, Kansara A. Hypothyroidism and the Heart. Methodist Debakey Cardiovasc J 2017; 13: 55-59. https://doi.org/10.14797/mdcj-13-2-55
- Hashim R, Shaheen S, Ahmad S, Sattar A, Khan FA. Comparison of serum creatine kinase estimation with short tandem repeats based linkage analysis in carriers and affected children of Duchenne muscular dystrophy. J Ayub Med Coll Abbottabad 2011; 23: 125-128.
- Sindoni A, Rodolico C, Pappalardo MA, Portaro S, Benvenga S. Hypothyroid myopathy: A peculiar clinical presentation of thyroid failure. Review of the literature. Rev Endocr Metab Disord 2016; 17: 499-519. https://doi.org/10.1007/s11154-016-9357-0
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160: 1573-1575. https://doi.org/10.1001/archinte.160.11.1573
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.