Graphical Abstract
Abstract
Objective. This study aimed to explore the distribution, trends, and clinical characteristics of various types of childhood diabetes, including type 1 diabetes (T1DM), type 2 diabetes (T2DM), and maturity-onset diabetes of the young (MODY) in a tertiary health center.
Methods. We conducted a comprehensive review of medical records of individuals aged 0–18 years who were diagnosed with diabetes between January 1996 and December 2023. Clinical and laboratory characteristics at the time of diagnosis, along with the specific diabetes type, were meticulously documented.
Results. A total of 1219 patients were included in the study, of whom 48.4% were female, with a mean age at diagnosis of 9.1 ± 4.3 years. T1DM was diagnosed in 85.8% of patients, T2DM in 6.3%, clinical MODY in 5.2%, and rare forms of diabetes in 2.6%. An increasing trend in T2DM and MODY cases has been observed since 2007. Diabetic ketoacidosis (DKA) was most prevalent in T1DM (47.1%), followed by T2DM (5.2%) and MODY (1.6%). Mean C-peptide levels at diagnosis were 0.57 ± 0.5 ng/mL in T1DM, 3.2 ± 1.3 ng/mL in T2DM, and 1.4 ± 0.9 ng/mL in MODY. Antibody positivity was observed in 78.8% of T1DM, 6.5% of T2DM, and 15.9% of MODY cases. Among the MODY group, genetic analysis was performed in 48 (75%) patients, with GCK gene mutations identified as the most common genetic abnormality in 27 (56.2%) of these patients.
Conclusion. This study demonstrates that T1DM is still the most commonly diagnosed type of diabetes in childhood, while T2DM and MODY are less frequent. However, a temporal increase in the incidence of MODY and T2DM subtypes was observed. The incidence of DKA at diagnosis was significantly higher in T1DM patients compared with those diagnosed with MODY or T2DM.
Keywords: type 1 diabetes, type 2 diabetes, monogenic diabetes, matury-onset diabetes of the young (MODY), childhood
Introduction
Recent trends have shown that the incidence of diabetes is increasing rapidly worldwide1, and there has also been a dramatic increase in its incidence in Turkish children.2,3 Type 1 diabetes mellitus (T1DM) is the most common type of diabetes in children and continues to increase in different parts of the world.1,4-6 Childhood obesity, which is increasing worldwide depending on ethnicity and country of residence, has been associated with a variable increase in the prevalence of type 2 diabetes (T2DM).7 The prevalence of T2DM in children has been reported to be 11% in the USA8, compared to 1.3% in Europe.9 In six regions of the USA, the prevalence of diabetes in children and adolescents has been reported to be increasing significantly for both type 1 and type 2 diabetes.10
Childhood T2DM can be confused with maturity-onset diabetes of the young (MODY) due to family history, presentation and a possible confounding factor of obesity/overweight.11 MODY can also be misclassified as T1DM, particularly due to HNF1A mutations.12 Determining the type of diabetes is important for therapeutic evaluation and genetic counselling.13 The most common type of monogenic diabetes is MODY, a clinically and genetically heterogeneous group of endocrine disorders affecting 1-5% of patients with diabetes.14 The increasing availability of molecular diagnostics has made it possible to identify other types of monogenic diabetes in addition to type 1 and type 2 diabetes with polygenic inheritance. In this classification, the concept of monogenic diabetes includes MODY, mitochondrial diabetes, Wolfram syndrome, neonatal diabetes and specific syndromes due to genetic defects causing insulin resistance.15
Until about 15 years ago, the distinction between T1DM, T2DM and MODY was somewhat simpler. However, with the increase in genetic studies and the definition of associations, it has become clear that these distinctions are not clear-cut. It has shown us that there are intertwined forms of diabetes. In daily practice, the differential diagnosis includes clinical findings, laboratory tests and diabetes-associated autoantibodies. This study will discuss these parameters in the differential diagnosis of diabetes.
The aims of this study were: (1) to evaluate the aetiological distribution and temporal changes of childhood diabetes, (2) to compare the clinical and laboratory characteristics of different types of diabetes seen in the pediatric diabetes centre of a tertiary care hospital during the last 28 years. The aim was also to contribute to the differential diagnosis of childhood diabetes subgroups.
Materials and Methods
The study was approved by the Ethics Committee of İnönü University (approval no: 2024/5706). Data of children and adolescents aged 0-18 years who were diagnosed with diabetes mellitus at İnönü University Turgut Özal Medical Center Training and Research Hospital in Malatya, Türkiye during the 28-year period between January 1996 and December 2023 were analyzed. Patients with a follow-up period of less than one year and those with undetermined diabetes type due to insufficient data were excluded from the study. In addition, because we could not clearly determine the number of patients who developed drug-associated diabetes, these patients were also excluded from the study. The observational and retrospective study included 1219 patients.
Sex, age at diagnosis, height, weight, body mass index (BMI), glucose, insulin, HbA1c, C-peptide levels at diagnosis, presence of symptoms, presence of diabetes-associated autoantibodies (islet cell antibodies, glutamic acid decarboxylase antibodies and insulin autoantibodies), presence of ketone bodies, pH at diagnosis and type of diabetes were recorded. The islet cell antibody (ICA), insulin autoantibody (IAA), and glutamic acid decarboxylase (GAD) levels were measured based on antigen-antibody detection using enzyme-linked immunosorbent assays with the Isletest kit in the Seac Brio 410499 model instrument. Patients were classified according to the ISPAD Consensus 2022 (Table I).16 Clinical findings were queried as polyuria, polydipsia, polyphagia and weight loss, and patients with one of these four clinical findings were considered symptomatic. Standard deviation scores (SDS) for height, weight, and BMI were calculated based on reference data from healthy Turkish boys and girls.17
|
*Column percentages. **The development of diabetes following surgery for hyperinsulinemic hypoglycemia. DM: diabetes mellitus; IPEX: immune dysregulation, polyendocrinopathy, enteropathy, X-linked; MODY: maturity onset diabetes of the young, TRMA: thiamine-responsive megaloblastic anemia. |
||
| Table I. Distribution of diabetic patients according to subgroups | ||
|
|
|
|
| Type 1 diabetes mellitus |
|
|
| Type 2 diabetes mellitus |
|
|
| MODY |
|
|
| Neonatal DM |
|
|
| Wolfram syndrome |
|
|
| Diseases of the exocrine pancreas |
|
|
| Alström syndrome |
|
|
| Mitochondrial DM |
|
|
| Genetic defect in insulin action |
|
|
| TRMA |
|
|
| Subtotal pancreatectomy** |
|
|
| IPEX syndrome |
|
|
| Woodhouse-Sakati syndrome |
|
|
| Prader-Willi syndrome |
|
|
| Generalized lipodystrophy |
|
|
| Total |
|
|
T1DM was diagnosed based on the presence of severe insulin deficiency, diabetes-associated autoantibodies positivity, and the absence of any clinical signs suggestive of alternative causes of diabetes. The diagnostic criteria for T2DM included overweight or obesity, clinical features of insulin resistance (such as acanthosis nigricans, hypertension, and dyslipidemia), a family history of T2DM, and good metabolic control achieved with metformin alone, or with metformin combined with a low dose of long-acting insulin (<0.5 U/kg/day).7,15,16 Patients with a family history of diabetes for at least two generations on one side of the family, negative diabetes-associated autoantibodies, no evidence of insulin resistance and good metabolic control with diet, sulfonylurea or low-dose insulin were clinically classified as MODY. HNF1A, HNF4A, GCK and other genes have been analysed in clinically suspected cases of MODY, and a MODY panel has been studied in recent years. To differentiate between mutations and polymorphisms among the detected variants, segregation analyses were conducted within families. In silico analysis tools, including Provean, SIFT (Sorting Intolerant From Tolerant), PolyPhen, Franklin, and MutationTaster, were employed to evaluate the potential pathogenicity of the variants. In accordance with the 2015 ACMG guidelines18, variants were classified as “pathogenic”, “likely pathogenic”, “variant of uncertain significance (VUS)”, “likely benign” or “benign”. Individuals carrying variants classified as “pathogenic” or “likely pathogenic” based on in silico analyses were considered to have monogenic diabetes. Children with onset of diabetes before the age of six months were diagnosed with neonatal diabetes mellitus (NDM) and appropriate genetic testing was performed.
Statistical analysis
All statistical data were analysed using SPSS statistical software for Windows, version 21.0 (SPSS, Chicago, IL, USA). Variables were calculated using descriptive statistics. Data were presented as mean ± standard deviation (SD) or median (interquartile range, IQR, Q1-Q3). Normality was assessed using the Kolmogorov-Smirnov test. Parametric and non-parametric tests were used for comparisons between groups. The chi-square test was used for categorical variables. Student’s t-test was used for continuous variables in independent groups. Mann-Whitney U test was used for continuous variables that were not normally distributed. In statistical analyses, p < 0.05 is the accepted threshold for significance.
Results
The mean age at diagnosis of 1219 patients (48.4% female) was 9.1±4.3 (range 0.0-18.0) years. 23.1% (n=242) of patients with T1DM were younger than 5 years. 1046 patients were diagnosed with T1DM (85.81%), 77 patients with T2DM (6.31%), 64 patients with MODY (5.25%) and 32 patients with other types of diabetes (2.62%) (Table I). Seven of the cases were definitively diagnosed with NDM. During follow-up, four cases were classified as transient NDM, while three cases were confirmed as permanent NDM. The clinical characteristics at diagnosis of T1DM, T2DM and MODY are compared in Table II. The median age at diagnosis was 8.6 years (IQR: 5.1-12.0) for T1DM, 15 years (12.6-16.2) for T2DM, and 10.9 years (7.2-13.0) for MODY. A single case of T2DM diagnosed before the age of 10 years was identified. This patient, a 9.8-year-old girl at the time of diagnosis, also presented with precocious puberty. Positivity for at least one diabetes-associated autoantibody was 78.8% in T1DM, 6.5% in T2DM and 15.9% in MODY. IAA positivity before the age of 5 years was significantly higher in T1DM cases than > 5 years (41.1% vs. 32.6%, p=0.045).
|
Data were presented as n (%), mean±standard deviation, or median (Q1-Q3). *The n values represent the number of patients for whom data was available. **T1DM: 198, T2DM: 74, MODY: 62. aChi-square test. bIndependent samples t test. cMann-Whitney U test. In statistical analyses, p<0.05 is the accepted threshold for significance. BMI: body mass index, DKA: diabetic ketoacidosis, F: female, GAD: glutamic acid decarboxylase, HbA1c: hemoglobin A1c, IAA: insulin autoantibody, ICA: islet cell antibody, M: male, MODY: maturity onset diabetes of the young, SDS: standard deviation score, T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus. |
|||||||
| Table II. A comparative analysis of metabolic and clinical characteristics of patients diagnosed with type 1 diabetes, type 2 diabetes, and maturity-onset diabetes of the young at time of diagnosis. | |||||||
|
|
|
|
|
|
|
|
|
| Sex (F/M), n |
|
|
|
|
|
|
|
| Age at diagnosis (year) |
|
|
|
|
|
|
|
| Height SDS |
|
|
|
|
|
|
|
| Weight SDS |
|
|
|
|
|
|
|
| BMI SDS |
|
|
|
|
|
|
|
| BMI SDS ≥2 |
|
|
|
|
|
|
|
| BMI SDS <2 |
|
|
|
|
|
|
|
| Presence of symptoms |
|
|
|
|
|
|
|
| Parental consanguinity |
|
|
|
|
|
|
|
| Age <5 years |
|
|
|
|
|
|
|
| Fasting glucose (mg/dL) |
|
|
|
|
|
|
|
| Insulin (µU/mL) |
|
|
|
|
|
|
|
| C-peptide (ng/mL) |
|
|
|
|
|
|
|
| HbA1c (%) |
|
|
|
|
|
|
|
| pH |
|
|
|
|
|
|
|
| DKA |
|
|
|
|
|
|
|
| Ketosis |
|
|
|
|
|
|
|
| Hyperglycemia |
|
|
|
|
|
|
|
| Antibody positivity |
|
|
|
|
|
|
|
| Anti-GAD positivity |
|
|
|
|
|
|
|
| ICA positivity |
|
|
|
|
|
|
|
| IAA positivity |
|
|
|
|
|
|
|
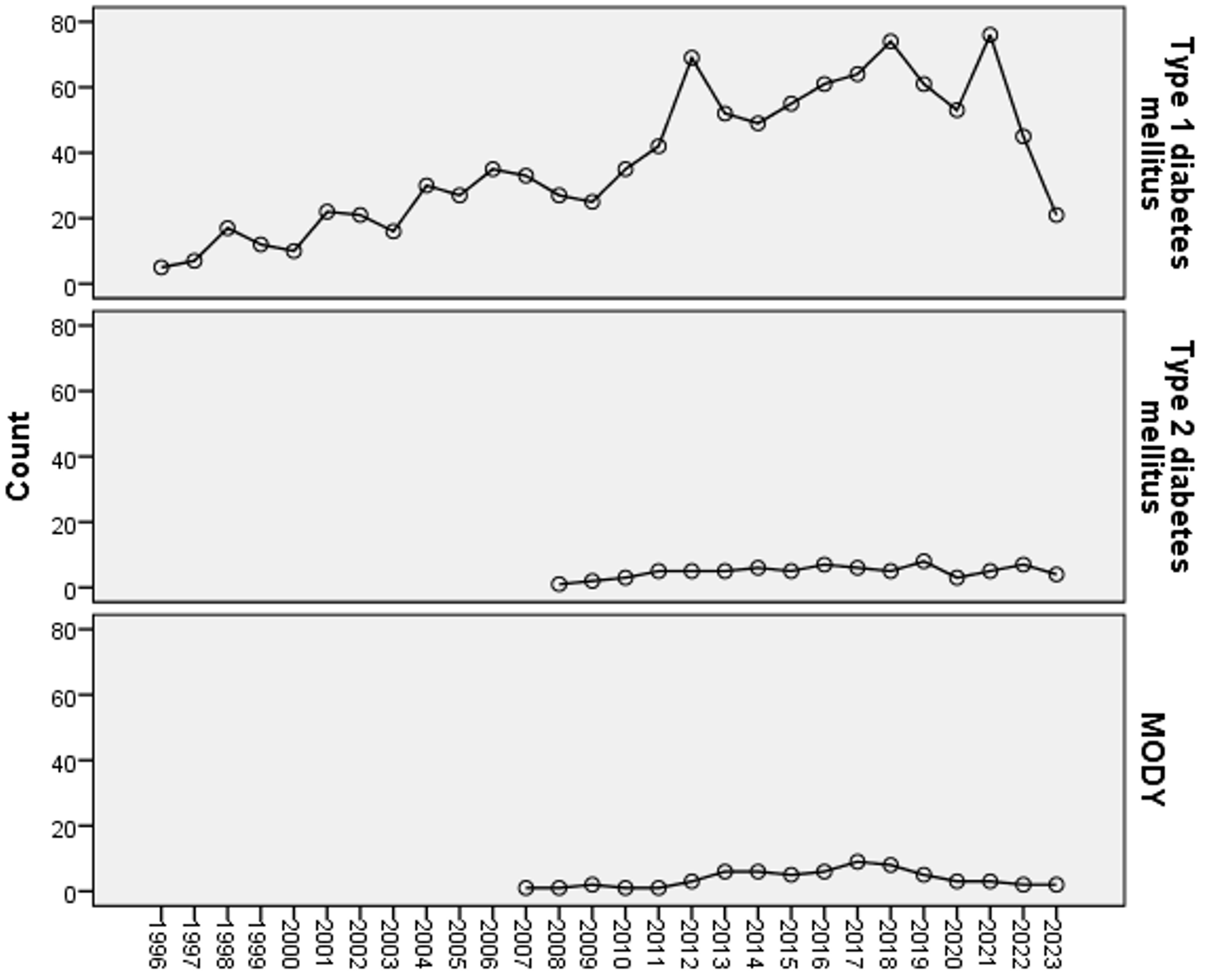
T2DM and MODY cases started to be seen in the cohort from 2007 onwards, this increasing trend over time is shown in Fig. 1. T1DM, on the other hand, seemed to increase over time, but the number of type 1 diabetes cases decreased in 2023. The prevalence of diabetic ketoacidosis (DKA) in T1DM at diagnosis was 47.1%, 47.8% in patients <5 years and 44.8% in patients >5 years (p=0.486). The prevalence of DKA decreased to 61.5% between 1996-2002, 51.0% between 2003-2009 and 32.9% between 2010-2016. However, it increased again between 2017 and 2023, reaching 41.2%. At the time of diagnosis, 62 (81.6%) patients with T2DM had hyperglycemia, ten (13.2%) had ketosis and only four (5.2%) had DKA (Table I).
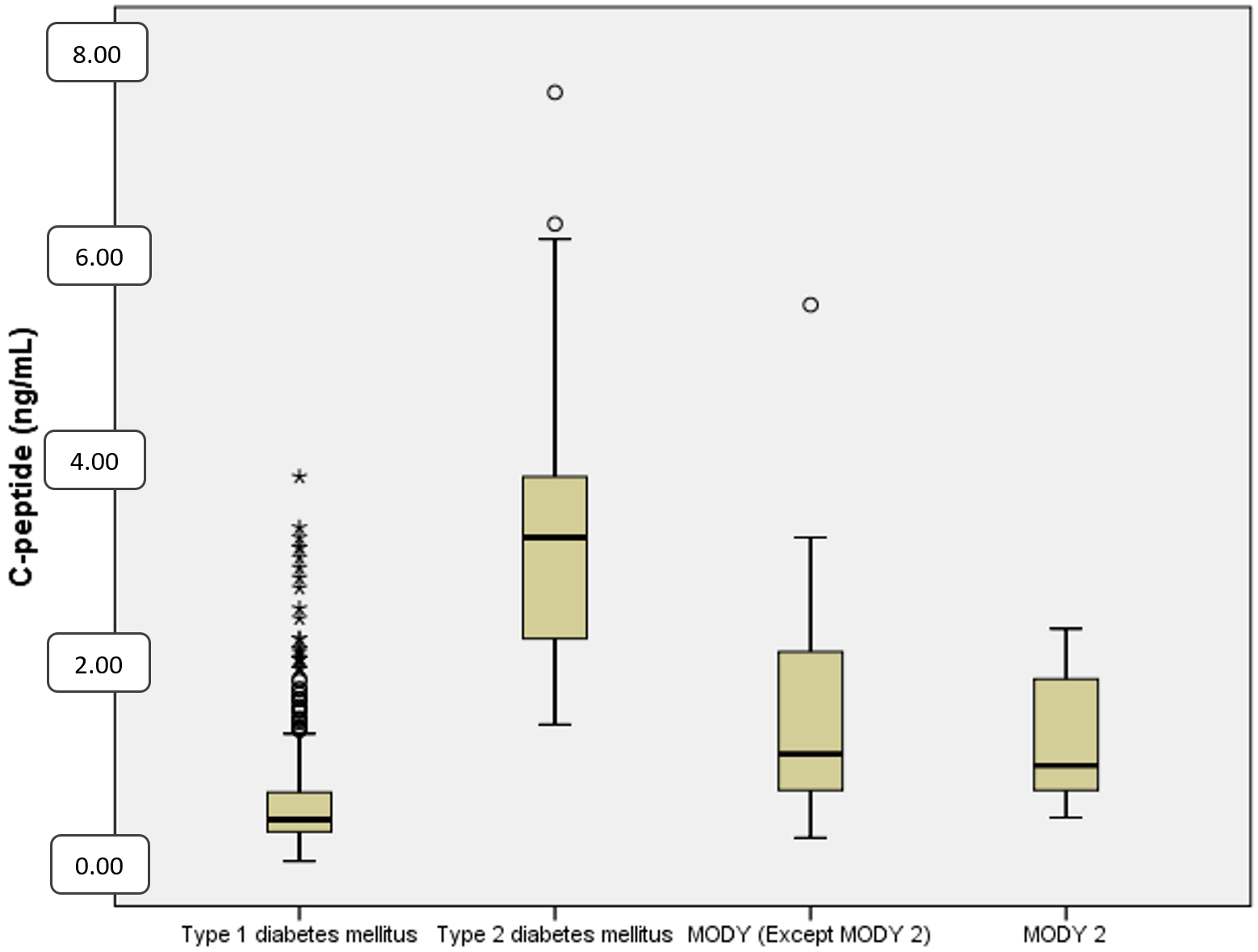
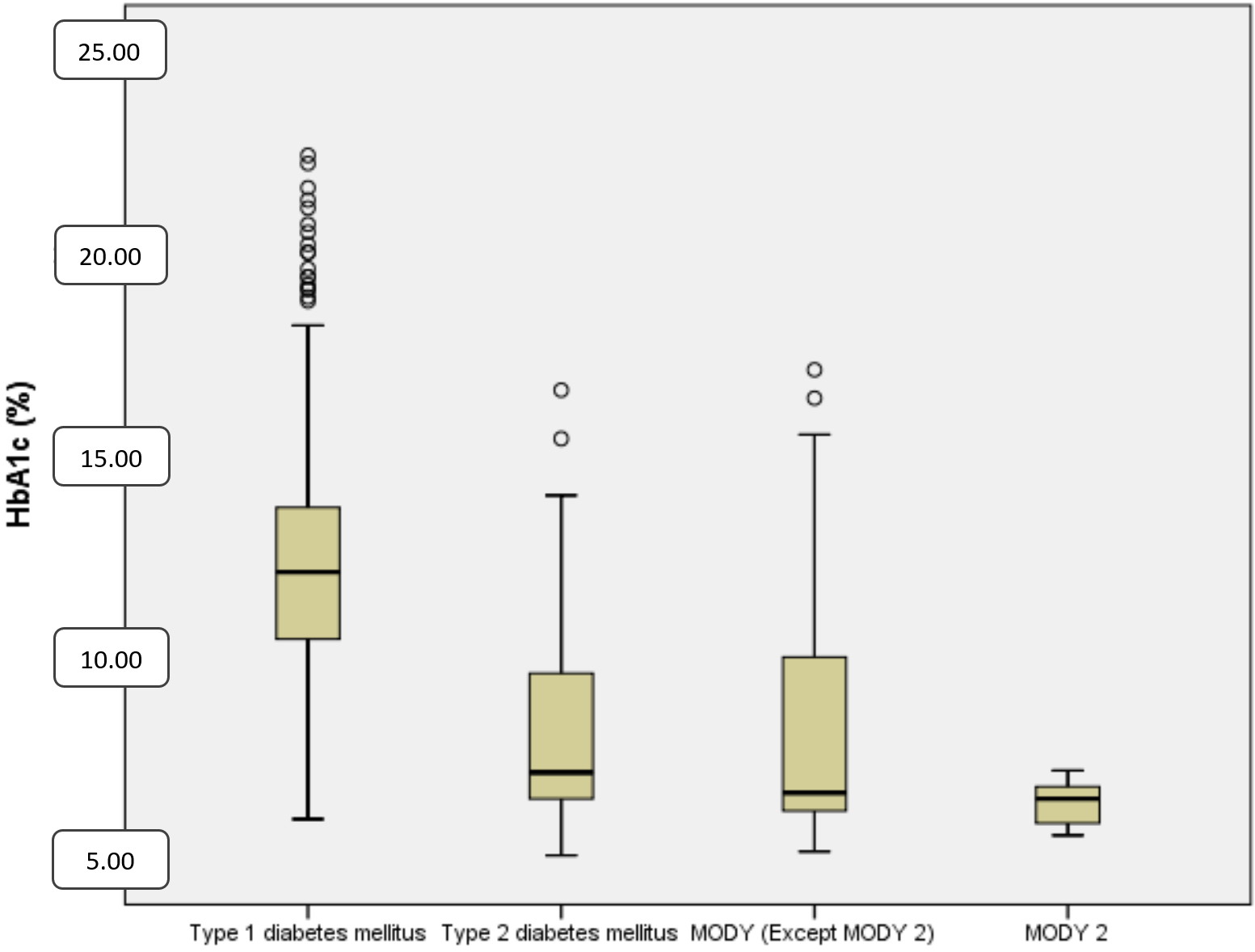
Mean C-peptide levels at diagnosis were 0.57±0.5 ng/mL in T1DM, 3.2±1.3 ng/mL in T2DM and 1.4±0.9 ng/mL in MODY patients (p<0.001) (Table II and Fig. 2). The mean HbA1c level at diagnosis was 12.2% ± 2.6% in T1DM, 8.1% ± 2.3% in T2DM and 7.6% ± 2.7% in MODY patients. HbA1c levels were significantly higher in T1DM (p<0.001) (Table II and Fig. 3).
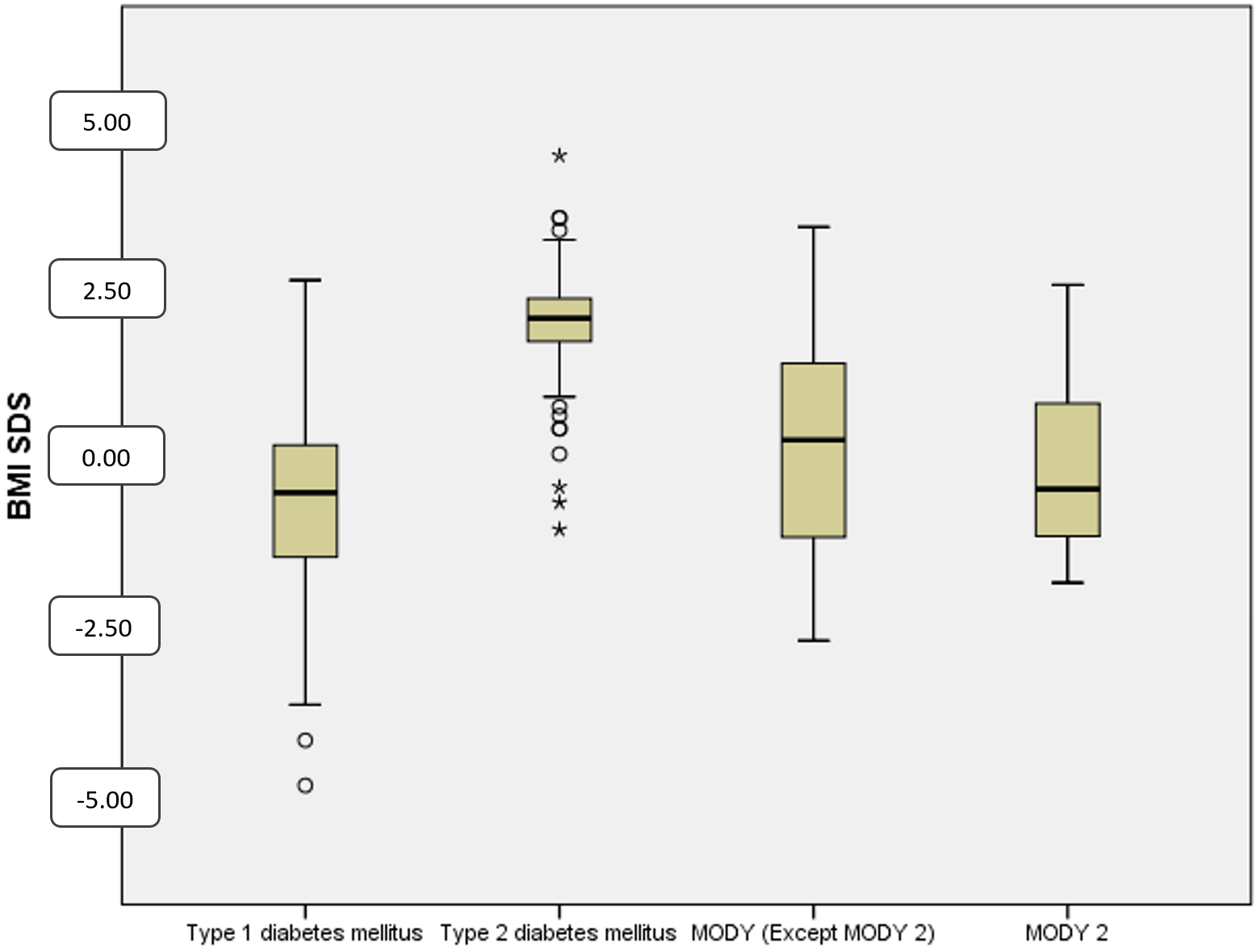
The median BMI-SDS at diagnosis was –0.5 (–1.5-0.1) in T1DM, 2.0 (1.6-2.3) in T2DM, and –0.2 (–1.2 -1.1) in MODY patients. It was found to be significantly higher in T2DM (p<0.001). Obesity was present 2% of T1DM, 10.9% of MODY and 93.7% of T2DM patients. Malnutrition was found in 13.6% of T1DM patients, 9.7% of MODY patients and was absent in T2DM patients (Table II and Fig. 4). Genetic analysis was available in 48 (75%) of 64 clinical MODY patients, and GCK mutations were detected in 27 (56.2%) patients.
Discussion
In the present study, we analysed the prevalence of diabetes subgroups in our population and these data allowed us to make comparisons with the literature. The overall prevalence of T1DM, T2DM, MODY and other types of diabetes was 85.81%, 6.31%, 5.25% and 2.62%, respectively. T1DM is still the most common cause of diabetes in children and its prevalence varies from 83-95% in different parts of the World.5,6,8,9,12,19 This difference is due to the number of children with T2DM and MODY. In the SEARCH study (USA, multicenter), the prevalences of T1DM, T2DM and MODY were 85.6%, 10.8% and 1.2%, respectively, whereas in the SWEET study (48 participating centers, 37 from Europe) these rates were 95.5%, 1.3% and 1.5%, respectively.9,20 In the SEARCH studies, the burden of T1DM was highest among non-Hispanic White youth, whereas the burden of T2DM was greatest among minority youth, particularly among American Indian and Alaska Native populations.8,20 The variation in frequencies may be explained by the accessibility of genetic testing and also by the prevalence of obesity in this region. In this study, the diagnoses of T2DM (6.3%) and MODY (5.2%) were more common than in SWEET. As this was not a national multicentre study, it is thought that this difference may be explained by the referral of rare types of diabetes to our tertiary care centre and easier access to genetic testing.
The country with the highest incidence of T1DM in childhood is Finland, followed by Sweden. Recent studies show an increasing trend in the Arabian Gulf countries and Türkiye.1-6 Previously, we found an annual increase of 8.3% in the incidence of T1DM in our centre.2 The incidence of T2DM, thought to be rare in children, is gradually increasing, and in parallel, the incidence of obesity in children is increasing, making it difficult to clinically differentiate between different types of diabetes.5,6 In studies of MODY in children, misdiagnosis of type 1 or type 2 diabetes has been observed.5 Recently, the number of cases diagnosed with MODY has been increasing with increasing awareness and molecular diagnostic capabilities.
The most important and accessible data in the differential diagnosis of diabetes subgroups are clinical findings (polyuria, polydipsia, polyphagia, and weight loss), glucose, insulin, C-peptide, HbA1c and the presence of diabetes-associated autoantibodies. Polyuria, polydipsia, polyphagia and weight loss are more suggestive of T1DM, but can also be seen in MODY.21 Clinical findings indicate the severity of insulin deficiency.21 In our study, the clinical findings were 93.7% in T1DM, 37.1% in T2DM and 35.4% in MODY. In T1DM, clinical findings are the most important.
One of the most confusing clinical findings in diabetes classification is obesity. At the time of diagnosis, BMI is considered to be a less determinant feature in classification. This is because the increase in obesity has led to the emergence of obese children with T1DM and MODY.22 Although different studies have used different criteria, the prevalence of obesity at the time of diagnosis in T1DM patients varies from 3.1% to 9%.11,12 In this study, obesity was found in 2.0% of patients with T1DM. This may be due to the lower rates of obesity in our pediatric population compared to North America and Western Europe.23 Consistent with previous reports, T2DM is more common in girls and in adolescents.14,15,23 In this study, the youngest documented case of T2DM was that of a 9.8-year-old girl who presented with precocious puberty. No additional cases of T2DM were identified in children under the age of 10 years, the mean age at diagnosis was 14.4 years, and almost all cases were in the adolescent age group. MODY can occur at any age.
While glucose and HbA1c are very effective in diagnosis, they are not very helpful in differential diagnosis. While HbA1c is generally below 7.5% in MODY 2 and 8-9% in other MODYs, this rate is above 10% in type 1 diabetes.21 Similar results were found in our study; in general, HbA1c is higher in T1DM. In diagnosis, C-peptide level is more valuable than insulin level in demonstrating reserve, and a C-peptide level >1.2 ng/mL in diagnosis suggests T2DM or MODY.24 In our study, mean C-peptide level was 0.57 ng/mL and significantly low in T1DM, high in T2DM and moderate in MODY. In this study, there was little overlap in C-peptide levels at the time of diagnosis, especially in T1DM and T2DM, which helped us to differentiate these two types of diabetes. It also led us to distinguish T2DM from MODY, albeit weakly.
Antibody positivity in T2DM has been reported up to 15% and these antibody positive patients tend to be younger, less overweight/obese and have higher HbA1c levels.25Therefore, different terms such as type 1.5 diabetes, double diabetes and occult autoimmune juvenile diabetes have been used. In the present study, antibody positivity in T2DM was found to be 6.5%. In T2DM patients aged 10 years and older, antibody positivity was reported in 9.8% in the TODAY study26 and 21.2% in the SEARCH study.27 The diagnosis of this group of seropositive patients is controversial. Studies have shown antibody positivity in 1% of people with MODY.20 In the present study, 15.9% of our MODY patients had at least one antibody positivity. We were unable to explain the high antibody positivity observed in MODY patients. Therefore, in this study, antibody positivity was not used as an exclusion criterion for MODY.
In studies conducted in developed countries, the prevalence of DKA at the time of diagnosis of type 1 diabetes is reported to be 20-43%, whereas in the Arabian Peninsula these rates can be as high as 80%.28,29 In different studies conducted in Türkiye, the prevalence of DKA in T1DM at diagnosis varies between 44% and 65%.6,30-33 In our study, the rate of DKA was 47.1% and the current rate is still very high. The prevalence of DKA in pediatric T2DM at the time of diagnosis is variable, ranging from 4% to 40%.6,25,34 This rate was not high in our study (5.2%). The rate of DKA was lower in our MODY patients (1.6%). Ketosis without acidosis was found in 13% of our T2DM patients and 12.5% of our MODY patients. More than 80% of patients with T2DM and MODY were diagnosed after detection of hyperglycemia without ketosis or diabetic ketoacidosis. The most likely reason for this is that our centre is a tertiary care centre and we perform oral glucose tolerance test in all obese children with risk factors.
The frequency of MODY in children with diabetes varies from 1% to 6% in different studies.6,9,12,21 In studies reporting a higher frequency of MODY, GCK mutation is the most common cause.6,12,21 Similarly, we found a GCK mutation in 56.2% of genetically tested clinical MODY patients, which may be explained by the widespread use of random glucose measurement in general pediatric clinics in Türkiye. On the other hand, since genetic analysis was not available before 2010 and not all MODY genes could be analysed, the rate of genetic analysis in clinical MODY patients in this study was relatively low (75%). A mutation in one of the known MODY genes was found in 75% of these patients. This rate varies between 27-89% in different studies.6,21,35 The reasons why not all patients in our study received a genetic diagnosis may be due to the inability to detect mutations, inability to perform a MODY gene panel in all patients, inclusion criteria for genetic testing, mutation in a gene not yet identified, or diagnostic overlap of different types of diabetes.
Study limitations
This study has some limitations; (1) data from a tertiary care centre, (2) specifically, prior to 2010, the diagnosis of MODY was only made clinically, (3) not all patients with MODY after 2010 could be interviewed, (4) the frequency of some specific types of diabetes such as cystic fibrosis-related diabetes (CFRD) may not be the actual frequency, (5) cases with drug-induced diabetes could not be included in the study.
Conclusion
This study examines trends in pediatric diabetes over the past 28 years in a large patient population from a large city with access to a tertiary care diabetes centre. It aimed to identify the distinguishing characteristics of different types of diabetes in children. While the prevalence of T2DM is increasing among the paediatric population in Türkiye, it remains lower than reported rates in North America and Western Europe. In recent years, the recognition of MODY has increased, largely due to the wider availability of autoantibody testing and, more importantly, genetic testing. Although overlapping clinical features—such as obesity, ketosis, and autoantibody positivity—can complicate the differential diagnosis, demographic (e.g., age, sex, pubertal status, consanguinity) and laboratory parameters (e.g., autoantibody measurements, C-peptide levels, HbA1c) are valuable tools for accurately classifying diabetes types in the paediatric population.
Ethical approval
The study was approved by the local Ethical Committee of İnönü University (approval no: 2024/5706).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Harding JL, Wander PL, Zhang X, et al. The incidence of adult-onset type 1 diabetes: a systematic review from 32 countries and regions. Diabetes Care 2022; 45: 994-1006. https://doi.org/10.2337/dc21-1752
- Dündar İ, Akıncı A, Çamtosun E, Kayaş L, Çiftçi N, Özçetin E. Type 1 diabetes incidence trends in a cohort of Turkish children and youth. Turk Arch Pediatr 2023; 58: 539-545. https://doi.org/10.5152/TurkArchPediatr.2023.23036
- Esen I, Okdemir D. Trend of type 1 diabetes incidence in children between 2009 and 2019 in Elazig, Turkey. Pediatr Diabetes 2020; 21: 460-465. https://doi.org/10.1111/pedi.12984
- Ogle GD, James S, Dabelea D, et al. Global estimates of incidence of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res Clin Pract 2022; 183: 109083. https://doi.org/10.1016/j.diabres.2021.109083
- Haris B, Saraswathi S, Al-Khawaga S, et al. Epidemiology, genetic landscape and classification of childhood diabetes mellitus in the State of Qatar. J Diabetes Investig 2021; 12: 2141-2148. https://doi.org/10.1111/jdi.13610
- Haliloğlu B, Abalı S, Buğrul F, et al. The distribution of different types of diabetes in childhood: a single center experience. J Clin Res Pediatr Endocrinol 2018; 10: 125-130. https://doi.org/10.4274/jcrpe.5204
- Serbis A, Giapros V, Kotanidou EP, Galli-Tsinopoulou A, Siomou E. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes 2021; 12: 344-365. https://doi.org/10.4239/wjd.v12.i4.344
- Pettitt DJ, Talton J, Dabelea D, et al; SEARCH for Diabetes in Youth Study Group. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care 2014; 37: 402-408. https://doi.org/10.2337/dc13-1838
- Pacaud D, Schwandt A, de Beaufort C, et al. A description of clinician reported diagnosis of type 2 diabetes and other non-type 1 diabetes included in a large international multicentered pediatric diabetes registry (SWEET). Pediatr Diabetes 2016; 17(Suppl 23): 24-31. https://doi.org/10.1111/pedi.12426
- Lawrence JM, Divers J, Isom S, et al; SEARCH for Diabetes in Youth Study Group. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA 2021; 326: 717-727. Erratum in: JAMA 2021; 326: 1331. https://doi.org/10.1001/jama.2021.11165
- Kleinberger JW, Copeland KC, Gandica RG, et al. Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: the TODAY clinical trial. Genet Med 2018; 20: 583-590. https://doi.org/10.1038/gim.2017.150
- Delvecchio M, Mozzillo E, Salzano G, et al; Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetes (ISPED). Monogenic diabetes accounts for 6.3% of cases referred to 15 Italian pediatric diabetes centers during 2007 to 2012. J Clin Endocrinol Metab 2017; 102: 1826-1834. https://doi.org/10.1210/jc.2016-2490
- Redondo MJ, Hagopian WA, Oram R, et al. The clinical consequences of heterogeneity within and between different diabetes types. Diabetologia 2020; 63: 2040-2048. https://doi.org/10.1007/s00125-020-05211-7
- Nkonge KM, Nkonge DK, Nkonge TN. The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin Diabetes Endocrinol 2020; 6: 20. https://doi.org/10.1186/s40842-020-00112-5
- Hattersley AT, Greeley SAW, Polak M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes 2018; 19(Suppl 27): 47-63. https://doi.org/10.1111/pedi.12772
- Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 2018; 19(Suppl 27): 7-19. https://doi.org/10.1111/pedi.12773
- Neyzi O, Bundak R, Gökçay G, et al. Reference values for weight, height, head circumference, and body mass index in Turkish children. J Clin Res Pediatr Endocrinol 2015; 7: 280-293. https://doi.org/10.4274/jcrpe.2183
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405-424. https://doi.org/10.1038/gim.2015.30
- Libman I, Haynes A, Lyons S, et al. ISPAD Clinical Practice Consensus Guidelines 2022: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 2022; 23: 1160-1174. https://doi.org/10.1111/pedi.13454
- Pihoker C, Gilliam LK, Ellard S, et al. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for diabetes in youth. J Clin Endocrinol Metab 2013; 98: 4055-4062. https://doi.org/10.1210/jc.2013-1279
- Carlsson A, Shepherd M, Ellard S, et al. Absence of islet autoantibodies and modestly raised glucose values at diabetes diagnosis should lead to testing for MODY: lessons from a 5-year pediatric Swedish national cohort study. Diabetes Care 2020; 43: 82-89. https://doi.org/10.2337/dc19-0747
- Shields BM, Peters JL, Cooper C, et al. Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature. BMJ Open 2015; 5: e009088. https://doi.org/10.1136/bmjopen-2015-009088
- Alper Z, Ercan İ, Uncu Y. A Meta-analysis and an evaluation of trends in obesity prevalence among children and adolescents in Turkey: 1990 through 2015. J Clin Res Pediatr Endocrinol 2018; 10: 59-67. https://doi.org/10.4274/jcrpe.5043
- Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med 2013; 30: 803-817. https://doi.org/10.1111/dme.12159
- Zeitler P. Approach to the obese adolescent with new-onset diabetes. J Clin Endocrinol Metab 2010; 95: 5163-5170. https://doi.org/10.1210/jc.2010-0958
- Klingensmith GJ, Pyle L, Arslanian S, et al; TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care 2010; 33: 1970-1975. https://doi.org/10.2337/dc10-0373
- Writing Group for the SEARCH for Diabetes in Youth Study Group; Dabelea D, Bell RA, D’Agostino RB Jr, et al. Incidence of diabetes in youth in the United States. JAMA 2007; 297: 2716-2724. https://doi.org/10.1001/jama.297.24.2716
- Cherubini V, Grimsmann JM, Åkesson K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia 2020; 63: 1530-1541. https://doi.org/10.1007/s00125-020-05152-1
- Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 2012; 55: 2878-2894. https://doi.org/10.1007/s00125-012-2690-2
- Bilge Koca S, Zahit Takcı M, Deniz R, Özcan S, Çeleğen M, Dursun A. Change in the frequency of diabetic ketoacidosis in children with newly diagnosed type 1 diabetes in the Central Anatolia Region of Turkey over the years before and after the Coronavirus Disease 2019 pandemic: a single-center experience. Turk Arch Pediatr 2024; 59: 163-169. https://doi.org/10.5152/TurkArchPediatr.2024.23255
- Kandemir N, Vuralli D, Ozon A, et al. Epidemiology of type 1 diabetes mellitus in children and adolescents: a 50-year, single-center experience. J Diabetes 2024; 16: e13562. https://doi.org/10.1111/1753-0407.13562
- Dündar İ, Akıncı A, Camtosun E, Çiftçi N, Kayas L, Nalbantoğlu Ö. Trend in initial presenting features of type 1 diabetes mellitus over a 24 year period in Turkey: a retrospective analysis of 814 cases. Turk J Pediatr 2022; 64: 40-48. https://doi.org/10.24953/turkjped.2020.3580
- Özalkak Ş, Yıldırım R, Tunç S, et al. Revisiting the annual incidence of type 1 diabetes mellitus in children from the Southeastern Anatolian Region of Turkey: a regional report. J Clin Res Pediatr Endocrinol 2022; 14: 172-178. https://doi.org/10.4274/jcrpe.galenos.2021.2021-10-7
- Kubota-Mishra E, Huang X, Minard CG, et al; RADIANT Study Group. High prevalence of A-β+ Ketosis-Prone diabetes in children with type 2 diabetes and diabetic ketoacidosis at diagnosis: evidence from the Rare and Atypical Diabetes Network (RADIANT). Pediatr Diabetes 2024; 2024: 5907924. https://doi.org/10.1155/2024/5907924
- Irgens HU, Molnes J, Johansson BB, et al. Prevalence of monogenic diabetes in the population-based Norwegian Childhood Diabetes Registry. Diabetologia 2013; 56: 1512-1519. https://doi.org/10.1007/s00125-013-2916-y
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.