Graphical Abstract
Abstract
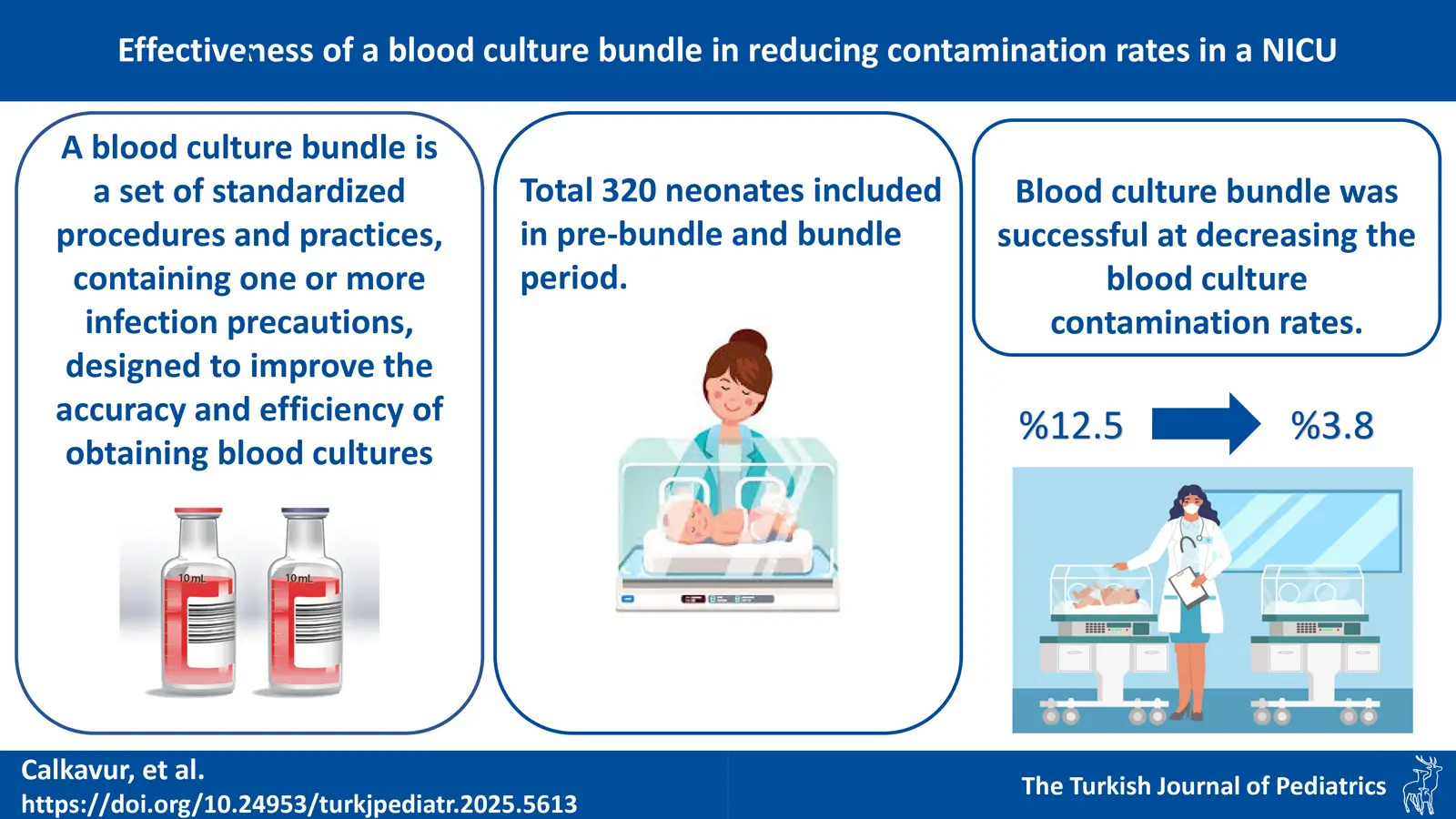
Background. Isolating microorganisms from blood cultures is the gold standard for identifying the cause of sepsis. However, contamination of the blood culture is a significant barrier to the blood culture’s utility. In this study, we aimed to evaluate the impact of blood culture bundles on the incidence of contamination in the neonatal intensive care unit (NICU).
Methods. A prospective research to compare pre-bundle and bundle periods was created. During the bundle period, a bundle for blood culture sampling was implemented. The numbers of unnecessary antibiotic days and hospital stay following a false positive blood culture were used to calculate costs.
Results. A total of 320 neonatal blood culture procedures were included. The rate of blood culture contamination was 3.8% in the bundle and 12.5% in the pre-bundle period, this was significantly higher in the pre-bundle period (p<0.001). The implementation of the blood culture bundle reduced blood culture contamination by 69.6%. The average number of hospital days attributed to blood culture contamination was 3.8 days. The average cost of a hospital stay due to contamination of one blood culture was $883.12. During the study, 14 blood culture contaminations, 54 unnecessary NICU stay days were avoided and $12549.6 were saved.
Conclusions. We found that the blood culture bundle program was successful at decreasing the blood culture contamination, preventing additional hospital stay and treatment costs in the NICU.
Keywords: blood culture bundle, blood culture contamination, cost-effectiveness; neonatal intensive care unit, neonates
Introduction
Infections are never as frequent and life-threatening as in the neonatal period. Neonatal sepsis is a clinical syndrome in which clinical signs and laboratory results of infection are present in the first month of life and a specific agent is produced in blood culture. Despite the advances in neonatology, it is still an important cause of morbidity and mortality. The incidence of neonatal sepsis is reported to be between 1-8.1 per 1000 live births and lower in developed countries.1The lack of sepsis-specific findings in the neonatal period, the narrow repertoire of clinical findings in newborns, and the fact that non-infectious clinical conditions frequently encountered in this period have similar findings pose a serious problem for early diagnosis and treatment of neonatal sepsis. Another important problem is that early empirical antibiotic treatment without diagnostic confirmation has to be resorted to very frequently in order to avoid morbidity and more importantly mortality with early intervention. This leads to unnecessary and prolonged treatment of newborns with potentially dangerous broad-spectrum antibiotics as well as prolonged hospitalizations and significant costs.2,3
The diagnosis of sepsis in the newborn is made by evaluating clinical and laboratory findings together. The gold standard is the detection of growth in blood culture. However, detection of growth in blood cultures in newborns does not always give very accurate results due to the difficulties of growth due to insufficient blood sample collection and high contamination rates.4,5 A blood culture contamination is defined as a microorganism that is supposed to be introduced into the culture during either specimen collection or processing and that is not pathogenic for the patient.6 The rate of contaminated blood culture in the neonatal period is 2.6-18%.7 There are numerous studies on practices to reduce blood culture contamination in children and neonates, including dedicated blood collection teams, application of different skin disinfection solutions, commercially produced blood culture collection packs, staff training programs, and improvements in hand hygiene.8-11
In this pre and post intervention study we aimed to evaluate the impact of blood culture bundles on the incidence of blood culture contamination rates in the neonatal intensive care unit (NICU).
Materials and Methods
This observational prospective pre-post intervention study was conducted in the NICU of the University of Health Sciences, İzmir Faculty of Medicine, Dr. Behçet Uz Pediatric Diseases and Surgery Training and Research Hospital between May 2022 and May 2023. The hospital is a 400-bed pediatric teaching hospital and the NICU is a 58-bed level 4 unit with approximately 1300 patients admitted annually, which is an advanced referral center for a very large patient population including neonatal cardiac surgery and neonatal surgery.
The study covered two periods: a pre-bundle phase (May–December 2022) and a post-bundle phase (January–May 2023), each lasting six months. The checklist for the blood culture contamination precaution package (Supplementary Table S1) was prepared using guidelines from the Turkish Society of Clinical Microbiology.12
Sample size: A priori power analysis was conducted to determine the adequacy of the number of cases by using Medcalc v.12.3.0 (MedCalc Software bvba, Broekstraat 52, 9030 Mariakerke, Belgium). As false positive blood culture rates were not known in our unit prior to the study, false positive culture rates were estimated at 5% based on published literature. With a power of 80% and α = 0.05, we aimed to include 160 infants before and after initiation of the intervention to demonstrate that the false positive blood culture rate decreased from 5% to 0.5% with the use of the blood culture bundle.13
The patients included in the study included a total of 320 (160 study, 160 control group) neonates up to 28 days postnatal and/or 44 weeks postconceptional who were hospitalized in the NICU and required diagnostic blood cultures at any time during hospitalization. Patients with antibiotic use within 48 hours before blood culture, patients with underlying skin problems and patients whose results could not be obtained were excluded from the study. Among the patients hospitalized in the NICU before the start of the study, patients with a similar gestational week with the newborns included in the study group were selected retrospectively and taken as the control group.
During both periods, blood culture collection adhered to an aseptic technique. For this purpose, hands were cleaned with antibacterial liquid soap (Klorhexin scrub antibacterial liquid soap 4% - Ekin Medical), the patient’s skin was cleaned with povidone iodine 10% solution (Betadix 10% solution- Natural Medical Pharma) using sterile gloves, waited at least 30 seconds and then povidone iodine was removed from the skin with 70% alcohol. Then, 1 mL of blood collected with a 22 gauge or larger needle was contained in a pediatric blood culture bottle (BACTEC PedPlus, BD Diagnostics, Maryland, USA) and sent to the laboratory. Each blood culture bottle was placed in the BACTEC FX automated system (BD Diagnostics, Maryland, USA). Identification and antimicrobial susceptibility testing of the bacteria was performed using the automated VITEK-2 COMPACT (bioMérieux, Marcy l’Etoile, France) system. Gram-positive bacteria identification and antimicrobial susceptibility testing were performed using the VITEK-2 AST-P664 card. Gram-negative bacteria identification and antimicrobial susceptibility testing were done with VITEK-2 cards AST-N420, AST-N423, and AST-N326. Yeast identification was performed using a VITEK-2 AST-YST card. Antifungal susceptibility of the yeasts was determined by broth microdilution method with Sensititre YeastOne plate (Thermo Fisher Diagnostics BV, Landsmeer, The Netherlands).
At the beginning of the blood culture bundle implementation period, all doctors and nurses in charge of blood culture collection were informed about all items related to the checklist (Supplementary Table S1) with the blood culture contamination precaution package and its necessity. During blood culture collection using the checklist, one health care worker (HCW) performed the blood culture collection and the second HCW guided and filled the checklist form. Every procedure was observed by a supervisor nurse.
The patients’ gender, gestational week, birth weight, postnatal age (in days), presence of sepsis clinic, C-reactive protein (CRP) results, and results of bacteria grown in blood cultures, if any, were obtained from file notes and electronic medical record database. The data were evaluated and recorded by three investigators and the investigators were blinded to patient information to protect patient confidentiality.
Statistics were performed with SPSS statistical software (version 22; SPSS, Chicago, IL, USA). Average age, gestational age, and age at the culture collection were expressed as median, interquartile range (IQR, Q1-Q3) and the number of hospital stays were expressed as mean±SD. Student’s t-test for dependent and independent groups was used for comparisons of normally distributed numerical data, and Mann-Whitney U tests were used for non-normally distributed numerical data. Chi-square test was used to analyze categorical data. A p-value < 0.05 was considered statistically significant.
Economical evaluation
For patients misclassified as having bloodstream infections, the number of unnecessary antibiotic days was recorded according to unit protocol. Direct medical care cost items were calculated from the hospital perspective using a combination of micro-costing technique (resource-based accounting method) and hospital list data. Attributable length of stay for hospital admission was considered as the span of days for the treatment of central line associated bloodstream infection (CLABSI). Based on the number of patients affected due to blood culture contamination and the number of unnecessary antibiotic days, the extra hospitalization fees of the patients were calculated as Social Security Institution (SSI) payment. The investigators recorded the costs first in Turkish Lira (TL) and converted them to USD ($), using the average exchange rate between TL to USD currency between 01 May 2022 and 01 May 2023 ($1 = 17.69 TL, 1 TL = $0.056).
The local ethical committee of the Behçet Uz Pediatric Diseases and Surgery Training and Research Hospital gave its approval to this investigation with the date and registration number 12.01.2023/797, and informed consent forms were obtained from each participant prior to enrollment.
Results
A total of 320 neonatal blood culture procedures were analyzed, with 160 in the pre-bundle (control) and 160 in the bundle (intervention) period.
Comparison of the study and control group
There were 112 (70%) male and 48 (30%) female patients in the study group and 89 (55.6%) male and 71 (44.4%) female patients in the control group, and the males were significantly higher in the study group (p=0.008). The average gestational age and birth weight were not different between study and control group (p>0.05). The average age at blood culture collection was 28.5 days in the study group and 6.7 days in the control group, and significantly longer in the study group (p<0.001). The demographic features are given in Table I.
| CRP, C-reactive protein; SD, standard deviation. | |||
| Table I. Demographic characteristics and results of the pre and post-bundle groups. | |||
|
|
|
|
|
| Gender, n (%) | |||
| Male |
|
|
|
| Female |
|
|
|
| Gestational age (day), mean ±SD (min-max) |
|
|
|
| Birth weight (gram), mean ±SD (min-max) |
|
|
|
| Age at culture collection (day), median (Q1-Q3) |
|
|
|
| CRP positivity, n (%) |
|
|
|
| Clinical appearance of sepsis, n (%) |
|
|
|
CRP levels were <0.5 mg/dL in 120 (75%) patients in the study group, and 131 (81.9%) in the control group, and no significant difference was present between the groups (p>0.05). Fifty-one (31.9%) patients in the study group and 37 (23.1%) patients in the control group had clinical signs of sepsis, showing no significant difference (p>0.05).
Isolated microorganisms and blood culture results
When the patients were evaluated according to their clinical conditions, CRP results and blood culture growth results; 102 (63.8%) patients in the study group and 101 (63.1%) patients in the control group were evaluated as true negative. Among the 26 patients with confirmed bloodstream infections, the most common isolated microorganisms were Klebsiella pneumoniae (n=8; 30.8%), coagulase negative staphylococci (CoNS) (n=4, 15.4%), Serratia marcescens (n=4, 15.4%), Candida albicans (n=3, 11.5%), Candida tropicalis (n=2, 7.7%), Escherichia coli (n=2, 7.7%), Staphylococcus aureus (n=2, 7.7%), and Acinetobacter baumannii (n=1, 3.8%).
All the detected blood culture contaminants were CoNS. The rate of blood culture contamination in the study group was 3.8% (n=6) and 12.5% (n=20) in the control group, and significantly higher in the control group (p<0.001). The implementation of the blood culture bundle decreased the blood culture contamination by 69.6%. Blood culture results and the number of unnecessary hospitalization days are given in Table II.
| Table II. Blood culture results and number of unnecessary hospitalization days. | |||
|
|
|
|
|
| Blood culture growth, n (%) |
|
|
|
| True negative, n (%) |
|
|
|
| True positive, n (%) |
|
|
|
| Suspected, n (%) |
|
|
|
| Contamination, n (%) |
|
|
|
Economical and clinical evaluation of additional burden of blood culture contamination
The number of hospital stay day attributable to blood culture contamination was 3.8±1.5 (range from 2 to 6) days. In Türkiye, NICU payment by the social security system is based on a fixed payment per day and was 411152 TL ($232.4) per patient day. Therefore the average cost of hospital stay attributable to contamination of one blood culture was calculated as $883.12 (ranging from minimum $464.8 to maximum $1394.4).
Within the implementation of the blood culture bundle, 14 blood culture contaminations were prevented during the study, preventing 54 unnecessary NICU stay days and saving $12549.6 in a 6-month period. In a model of NICU with an annual number of 1000 blood culture sampling, within the implementation of the blood culture bundle, a total of 87 per 1000 blood culture samples will be prevented. In this way, a minimum of 174 days, a maximum of 522 days, and an average of 330 days of unnecessary hospitalization due to blood culture contamination per 1000 blood cultures will be prevented. The total money saved within the implementation of the blood culture bundle will range from $40437.6-$12131.28 with an average of $76692 per 1000 blood culture sample. The cost savings that can be reduced by preventing blood culture contamination as a result of package application are given in Table III.
| NICU, Neonatal Intensive Care Unit; SD, standard deviation. | |
| Table III. Cost-savings that can be reduced by preventing contamination with the bundle implementation. | |
| Definition | |
| NICU pay per day by the social security system ($) | 232.4 |
| Total additional cost per contaminated blood culture ($), mean ±SD (min-max) | 883.12±268.35 (464.8-1394.4) |
| Savings with the implementation of the bundle ($) | 12549.6 |
| Number of days of hospitalization saved by prevention 87 contamination /1000 blood cultures, mean ±SD (min-max) | 330±100.5 days (174-522) |
| 1000 blood cultures / 87 contamination prevention Total Savings ($), mean ±SD (min-max) |
76692 ±23358.6 (40437.6-121312.8) |
Discussion
The neonatal period is the most common period in which sepsis occurs during infancy. Neonatal sepsis is a clinical condition whose definitive diagnosis is based on growth in blood culture and which requires hospitalization for diagnosis and treatment.14 In this pre and post-intervention study we aimed to evaluate the impact of the blood culture bundles on the incidence of blood culture contamination rates in the NICU, and with the implementation of a blood culture bundle the rate of blood culture contamination decreased significantly by 69.6%. The number of hospital stay attributable to blood culture contamination was 3.8±1.5 days and the average cost of hospital stay attributable to the contamination of one blood culture was calculated as $883.12. The Clinical and Laboratory Standards Institute recommends a blood culture contamination target of 3% or less.15 However, this rate is reported in a wide range of 2.85-9.1% in the literature.16,17 In neonates, due to the difficulties in obtaining blood cultures, these rates can be in a wide range of 2.6-18% and much higher.7,18-20
The collecting blood culture procedure consists of many steps such as hand hygiene, skin disinfection, blood culture bottle preparation, blood collection, and handling of samples to the laboratory. Improper practices that cause contaminated blood culture can occur in each of these steps. In order to reduce the contamination rates, many applications have been made that affect the various stages of the blood culture procedure. Bundle applications ensure that the correct practices are carried out at every stage of blood culture collection, while at the same time ensuring regular training repetition and awareness raising of personnel. This leads to an exponential increase in the power of these practices.20Hand disinfection and hygiene before blood culture collection is one of them.
As a result of increasing compliance with hand hygiene in a tertiary NICU, the contamination rate for CoNS was reduced from 4.2±2.4 to 1.9±1.8 per 1000 patient days.21Routine wearing of sterile gloves prior to blood culture collection has been shown to reduce contamination rates by 50% compared to wearing optional sterile gloves.22Staff training and awareness are also effective in reducing blood culture contamination rates. In a study in which a staff training intervention program was implemented in an intensive care unit (ICU), it was reported that monthly ICU blood culture contaminant rates during the intervention period were reduced to an average of 3.7% compared to 9.5% in the baseline period.10In another study, contamination rates were found to be statistically significant at 14 versus 5.6 per 100 blood cultures before and after planned training on what to look out for during the blood culture collection procedure in the ICU.23
Besides these efforts, a dedicated blood culture sampling bundle and checklist had an exponential effect on lowering the blood culture contamination, and our experience also supported the effectiveness of the blood culture bundle. In addition to all these single applications, the idea that the application of two or three combinations of these applications together theoretically reduces blood culture contamination rates more has brought blood culture contamination prevention package applications to the agenda. In a non-randomized study in which only sterile collection packs and hand hygiene and skin cleaning were standardized in the NICU, the false positive rate, which was 4.6% before the application, was reduced to 0.6% after this application.20 A 12-month quality improvement (QI) program that implemented a package of transfer, inoculation, skin antisepsis, aseptic pack and blood volume optimization to reduce the blood culture contamination rate by 50% in a neonatal unit resulted in a blood culture contamination rate reduction from 2% to 1%.24
Blood culture contamination results in unnecessary laboratory tests, inappropriate antimicrobial therapy, long-term antimicrobial resistance, as well as prolonged hospital stay and increased cost.25,26 The number of hospital stay attributable to blood culture contamination was 3.8 days. In addition to the negative effects of hospitalization on mother-infant bonding and maintenance of breastfeeding, the mandatory empirical use of antibiotics in treatment has undesirable consequences in terms of its negative effects on all systems of the newborn, whose maturation and reserves are extremely limited, and the resulting dysbiosis.27 In a study comparing nurses taking blood cultures without a standard protocol with nurses taking blood cultures with sterile kits using a special sterile collection kit and laboratory-trained phlebotomy teams taking blood cultures, contamination rates associated with usual care, sterile kits and phlebotomy teams were found to be 4.34%, 1.68% and 1.10%, respectively, and the annual net savings using sterile kit and phlebotomy team strategies compared to the first group were $483.219 and $288.980, respectively.28
In our study, with the implementation of the blood culture bundle, $12549.6 in a 6-month period was saved. In addition, when the blood culture number increases, the money saved would increase up to $12131.28 per 1000 blood culture sample. A previous study estimated annual cost saving of approximately £250.100 with implantation of blood culture bundle. In a study evaluating hospital costs for patients with negative, false positive and true positive blood culture results, an average additional cost of $8.720 per contamination event was reported, supporting our study.29
This study has certain limitations. First of all, despite being a prospective, pre and post-intervention study, it lacks the advantages of a randomized control study. The patients in the two periods were not homogenous regarding age. In addition, we measured the cost of blood culture contamination as a direct cost of hospital stay, however, we did not account for indirect costs, such as additional laboratory investigations.
In conclusion, we found that the blood culture bundle program was successful at decreasing the blood culture contamination, preventing additional hospital stay and treatment costs in the NICU.
Acknowledgements
We would like to thank the NICU nurses of Dr. Behçet Uz Pediatric Diseases and Surgery Training and Research Hospital who for their collaborative effort during data collection.
Ethical approval
The study was approved by Ethical Committee of the Dr. Behçet Uz Pediatric Diseases and Surgery Training and Research Hospital (date: 12.01.2023, number: 797).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Nizet V, Klein J. Bacterial sepsis and meningitis. In: Wilson C, Nizet V, Maldonado Y, Remington J, Klein J, editors. Infectious Diseases of the Fetus and Newborn Infant. 8 ed. Philadelphia: Elsevier Saunders; 2016: 217-271.
- Kim JY, Rosenberg ES. The sum of the parts is greater than the whole: reducing blood culture contamination. Ann Intern Med 2011; 154: 202-203. https://doi.org/10.7326/0003-4819-154-3-201102010-00010
- Tripathi N, Cotten CM, Smith PB. Antibiotic use and misuse in the neonatal intensive care unit. Clin Perinatol 2012; 39: 61-68. https://doi.org/10.1016/j.clp.2011.12.003
- Huang SY, Tang RB, Chen SJ, Chung RL. Coagulase-negative staphylococcal bacteremia in critically ill children: risk factors and antimicrobial susceptibility. J Microbiol Immunol Infect 2003; 36: 51-55.
- Hazen KC, Polage CR. Using data to optimize blood bottle fill volumes and pathogen detection: making blood cultures great again. Clin Infect Dis 2020; 70: 269-270. https://doi.org/10.1093/cid/ciz203
- Dargère S, Cormier H, Verdon R. Contaminants in blood cultures: importance, implications, interpretation and prevention. Clin Microbiol Infect 2018; 24: 964-969. https://doi.org/10.1016/j.cmi.2018.03.030
- McLaughlin LM, Inglis GDT, Hoellering AB, Davies MW. Relationship between blood culture collection method and proportion of contaminated cultures in neonates. J Paediatr Child Health 2013; 49: 105-108. https://doi.org/10.1111/jpc.12088
- Hall RT, Domenico HJ, Self WH, Hain PD. Reducing the blood culture contamination rate in a pediatric emergency department and subsequent cost savings. Pediatrics 2013; 131: e292-e297. https://doi.org/10.1542/peds.2012-1030
- Bamber AI, Cunniffe JG, Nayar D, Ganguly R, Falconer E. Effectiveness of introducing blood culture collection packs to reduce contamination rates. Br J Biomed Sci 2009; 66: 6-9. https://doi.org/10.1080/09674845.2009.11730236
- Alahmadi YM, McElnay JC, Kearney MP, et al. Tackling the problem of blood culture contamination in the intensive care unit using an educational intervention. Epidemiol Infect 2015; 143: 1964-1971. https://doi.org/10.1017/S0950268814003008
- El Feghaly RE, Chatterjee J, Dowdy K, et al. A Quality Improvement Initiative: reducing blood culture contamination in a children’s hospital. Pediatrics 2018; 142: e20180244. https://doi.org/10.1542/peds.2018-0244
- Society for Specialization in Clinical Microbiology. Clinical Specimen to Final Report Implementation Guide. September 2017. Ankara. ISBN: 978-605-84108-7-9.
- Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med 2007; 26: 3661-3675. https://doi.org/10.1002/sim.2832
- Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med 2018; 6: 223-230. https://doi.org/10.1016/S2213-2600(18)30063-8
- Clinical and Laboratory Standards Institute. Principles and procedures for blood culture. Approved Guidelines M47-A. 1st ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2007: 67.
- Norberg A, Christopher NC, Ramundo ML, Bower JR, Berman SA. Contamination rates of blood cultures obtained by dedicated phlebotomy vs intravenous catheter. JAMA 2003; 289: 726-729. https://doi.org/10.1001/jama.289.6.726
- Ramsook C, Childers K, Cron SG, Nirken M. Comparison of blood-culture contamination rates in a pediatric emergency room: newly inserted intravenous catheters versus venipuncture. Infect Control Hosp Epidemiol 2000; 21: 649-651. https://doi.org/10.1086/501708
- Silva HL, Strabelli TM, Cunha ER, Neres SF, Camargo LF, Uip DE. Nosocomial coagulase negative Staphylococci bacteremia: five year prospective data collection. Braz J Infect Dis 2000; 4: 271-274.
- Krajčinović SS, Doronjski A, Barišić N, Stojanović V. Risk factors for neonatal sepsis and method for reduction of blood culture contamination. Malawi Med J 2015; 27: 20-24. https://doi.org/10.4314/mmj.v27i1.6
- Hamilton LF, Gillett HE, Smith-Collins A, Davis JW. A Sterile collection bundle intervention reduces the recovery of bacteria from neonatal blood culture. Biomed Hub 2018; 3: 1-7. https://doi.org/10.1159/000486703
- Sharek PJ, Benitz WE, Abel NJ, Freeburn MJ, Mayer ML, Bergman DA. Effect of an evidence-based hand washing policy on hand washing rates and false-positive coagulase negative staphylococcus blood and cerebrospinal fluid culture rates in a level III NICU. J Perinatol 2002; 22: 137-143. https://doi.org/10.1038/sj.jp.7210661
- Kim NH, Kim M, Lee S, et al. Effect of routine sterile gloving on contamination rates in blood culture: a cluster randomized trial. Ann Intern Med 2011; 154: 145-151. https://doi.org/10.7326/0003-4819-154-3-201102010-00003
- Sánchez-Sánchez MM, Arias-Rivera S, Fraile-Gamo P, et al. Effect of a training programme on blood culture contamination rate in critical care. Enferm Intensiva (Engl Ed) 2018; 29: 121-127. https://doi.org/10.1016/j.enfi.2017.12.003
- Allen E, Cavallaro A, Keir AK. A Quality Improvement Initiative to reduce blood culture contamination in the neonatal unit. Pediatr Qual Saf 2021; 6: e413. https://doi.org/10.1097/pq9.0000000000000413
- Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect 2011; 77: 233-236. https://doi.org/10.1016/j.jhin.2010.09.033
- Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA 1991; 265: 365-369.
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev 2006; 19: 788-802. https://doi.org/10.1128/CMR.00062-05
- Self WH, Talbot TR, Paul BR, Collins SP, Ward MJ. Cost analysis of strategies to reduce blood culture contamination in the emergency department: sterile collection kits and phlebotomy teams. Infect Control Hosp Epidemiol 2014; 35: 1021-1028. https://doi.org/10.1086/677161
- Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line-associated bloodstream infections. Am J Infect Control 2015; 43: 1222-1237. https://doi.org/10.1016/j.ajic.2015.06.030
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















