Abstract
Background. Necrotizing enterocolitis (NEC) is a prevalent and challenging intestinal disease in premature infants, lacking a specific pathogen consistently associated with its occurrence. Effectively preventing and treating NEC to reduce mortality rates remains a significant contemporary challenge. The present study aimed to explore the correlation between microRNA-149 gene polymorphism and NEC in premature infants in a Chinese Han population.
Methods. The expression levels of serum miR-149 were determined using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Polymorphism detection of the miR-149 gene rs2292832 polymorphism was performed by polymerase chain reaction. Multivariate logistic regression analysis was employed to investigate the association between the rs2292832 polymorphism and risk factors for NEC in preterm infants.
Results. General clinical data were compared between 102 preterm infants diagnosed with NEC and 263 preterm infants without NEC. Significant differences were observed in gestational age and birth weight. However, no significant differences were found in antenatal steroid use, sex, or feeding patterns between the two groups. The expression level of serum miR-149 was significantly reduced in premature infants with NEC, and there were differences in the allele frequency of the miR-149 rs2292832 polymorphism between the NEC group and control group. Specifically, the T allele and TT genotype of rs2292832 were associated with an increased susceptibility to NEC. Furthermore, both gestational age and the rs2292832 polymorphism showed a significant association with NEC risk, with the rs2292832 polymorphism of miR-149 being identified as the most prominent risk factor for NEC development in preterm infants.
Conclusions. The rs2292832 gene polymorphism of miR-149 may potentially exert an influence on susceptibility to NEC.
Keywords: Necrotizing enterocolitis (NEC), Gene polymorphism, MiR-149, rs2292832
Introduction
Necrotizing enterocolitis (NEC) is a prevalent and challenging-to-predict inflammatory intestinal disease in newborns, resulting from the complex interplay of multiple factors. These factors encompass preterm birth, formula feeding, abnormal colonization of intestinal microbiota, intestinal mucosal ischemia, infection, and dysbiosis.1,2 Relevant statistical data indicate that NEC primarily affects premature and low-birth-weight infants. The incidence rate among preterm infants ranges from 5% to 7%, while it reaches between 4% and 13% in premature newborns weighing less than 1500 grams. Furthermore, NEC remains a leading cause of mortality.3-5 As a leading cause of mortality and morbidity in neonatal intensive care units, NEC exhibits a high mortality rate ranging from 15% to 30%.1,6 Additionally, children afflicted by this disease often encounter adverse prognostic factors such as neurodevelopmental delay, growth disorders, and intestinal dysfunction. These complications not only significantly escalate healthcare costs but also profoundly impact the long-term quality of life for these children.7 However, despite extensive research on clinical risk factors associated with NEC occurrence thus far no specific pathogen has been consistently linked to enterocolitis development. Our understanding regarding the pathogenesis of NEC remains limited. Relying solely on clinical risk factors fails to elucidate individual variations in susceptibility to or severity of NEC among preterm infants. This indicates the involvement of genetic risk factors in disease pathogenesis.8 The potential genetic predisposition for NEC is gradually gaining recognition with expectations that genetic investigations will provide novel insights into its pathogenesis.
MicroRNAs (miRNAs) are a class of small, highly conserved non-coding RNAs that play a crucial role in regulating various cellular and metabolic pathways by binding to target mRNAs and modulating the expression of candidate proteins. Single nucleotide polymorphisms (SNPs) within miRNA sequences or their binding sites can potentially disrupt the interaction between miRNAs and mRNA targets, leading to dysregulation of gene expression.9 Consequently, SNPs in miRNAs have been implicated in the pathogenesis of numerous diseases. Previous studies have demonstrated significant downregulation of miR-149 in preeclampsia placenta10, with lower levels correlating with disease severity and potential diagnostic utility for preeclampsia.11 MiR-149 is also involved in trophoblast cell proliferation, migration, and invasion12, while its reduced expression level in preeclampsia placenta regulates trophoblast cell behavior.13 Moreover, decreased levels of miR-149-5p contribute to impaired angiogenesis in HUVEC cells.14 Additionally, overexpression of miR-149-5p can alleviate brain ischemia/reperfusion (I/R) injury by targeting Notch2. Gene polymorphisms play a pivotal role in the pathogenesis of various diseases.15
The investigation of candidate genes associated with the occurrence and progression of NEC can provide insights into the molecular mechanisms underlying NEC. In the Korean population, it has been observed that the TT genotype (T>C, rs2292832) of miR-149 is linked to an increased risk of spontaneous abortion.16 Conversely, the C allele of rs2292832 in miR-149 may confer protection against gastric mucosal atrophy.17 Furthermore, in an Iranian population using a recessive inheritance model (TT/(TC+CC)), a significant association between the rs2292832 variant in miR-149 and colorectal cancer susceptibility has been identified.18 Additionally, studies have demonstrated decreased expression levels of serum miR-149-5p in patients with inflammatory bowel disease.19 However, the impact of the rs2292832 polymorphism site in miR-149 on preterm infants’ susceptibility to NEC remains unclear.
This study aimed to further investigate the impact of miR-149 rs2292832 polymorphism on NEC by detecting differences in genotype and allele frequencies between preterm infants with and without NEC, providing novel insights for early diagnosis and treatment of NEC.
Materials and Methods
Study participants
In this study, 102 preterm infants diagnosed with NEC and admitted to the neonatal intensive care unit (NICU) of Women and Children’s Hospital of Ningbo University were selected as the NEC group based on inclusion and exclusion criteria. Simultaneously, a total of 263 preterm infants admitted to our hospital’s NICU who did not develop NEC were selected as the control group. The NEC group included infants with gestational ages ranging from 28 to 36 weeks, while the control group included infants with gestational ages ranging from 28.3 to 36.6 weeks. General patient data including sex, gestational age, antenatal steroid use, birth weight, and feeding characteristics were recorded. The inclusion criteria specifically required: (1) Neonates younger than 28 days; (2) Clear diagnosis of NEC according to Bell staging diagnostic standards with a stage greater than II. Exclusion criteria mainly included: (1) Presence of congenital intestinal malformations; (2) Gastroenteritis; (3) Inherited metabolic disorders; (4) History of hypoxia or asphyxia; (5) Pneumonia, intestinal atresia, Hirschsprung’s disease or other conditions; (6) Incomplete medical records or death within a few hours after admission; (7) Sepsis. To exclude infants with sepsis, we identified cases based on clinical symptoms, signs, and laboratory findings. Specifically, if an infant exhibited fever or hypothermia, lethargy, tachypnea, tachycardia, and other clinical manifestations, along with laboratory evidence such as abnormal white blood cell counts (either increased or decreased), elevated C-reactive protein levels, and positive blood cultures, the case was classified as sepsis. Infants diagnosed with sepsis were excluded from the study because sepsis can significantly impact intestinal function and systemic inflammatory response, potentially confounding the accurate assessment of the relationship between miR-149 gene polymorphisms and NEC. To ensure a more precise investigation of the association between the target gene polymorphism and NEC, we opted to exclude these cases.
This study has been approved by the Women and Children’s Hospital of Ningbo University Medical Ethics Committee (Ethics No. 2019-ky-042, Ethics Approval Date: 2019-6-22) and informed consent forms have been signed by all newborn guardians. All procedures adhere to the principles outlined in the Helsinki Declaration.
Isolation of DNA and genotyping
After the diagnosis of NEC, peripheral blood samples (0.5 ml) were collected from infants in both the control group and NEC group within 24 hours of admission, and placed in EDTA anticoagulation tubes. Subsequently, the blood samples were stored in epoxy resin tubes and kept at -80°C. The control group underwent blood sampling during the 42-day routine physical examination. Blood samples from both groups were uniformly analyzed within one week following the blood draw. Total DNA extraction and PCR amplification were performed using the QIA amp DNA Blood Mini Kit 51104 (cat No. 51304) (Qiagen, Germany), while Primer 5.0 software was utilized for primer design. This study focused on a specific SNP of the miR-149 gene, namely rs2292832. The PCR reaction conditions were as follows: pre-denaturation was performed at 95°C for 3 minutes; denaturation was carried out at 94°C for 30 seconds; annealing occurred at 55°C for 30 seconds, with a total of 35 cycles; subsequently, extension took place at 72°C for 30 seconds, followed by a final extension step at the same temperature lasting for 10 minutes. The amplified products were identified through agarose gel electrophoresis. Sanger sequencing was employed to determine the sequence of the amplified products. Finally, SNP typing analysis was conducted using Seq-Man software.
Quantitative real-time PCR (RT-qPCR)
The total RNA of miR-149 was extracted individually, followed by reverse transcription into cDNA using the cDNA as a template for RT-qPCR amplification. Subsequently, the resulting cDNA was amplified using the Roche Light Cycler® 96 instrument and reverse transcription reagents. The expression level of miR-149 was analyzed by RT-qPCR, and the relative gene expression level was calculated using the 2−ΔΔCt formula to reflect the multiplex change of miR-149 gene expression. U6 served as an internal control for miR-149.
Statistical analysis
Data processing was performed using SPSS 16.0 statistical software in this study. The effect size analysis (input: effect size w = 0.3, alpha error probability = 0.05, power (1-beta error probability) = 0.95, df = 5) indicated that the required sample size for calculation was 220. Quantitative data were presented as mean ± standard deviation, while qualitative data were expressed as percentages (%). Student’s t-test was employed for comparing two groups of quantitative data, and the χ2 test was used for comparing two groups of qualitative data. Additionally, unconditional logistic regression analysis was conducted to investigate the association between gene polymorphisms and the susceptibility and severity of NEC. Statistical significance was defined as P < 0.050.
Results
The general clinical characteristics of NEC individuals
This study enrolled a total of 102 preterm infants diagnosed with NEC and 263 preterm infants admitted to the NICU without NEC. Our actual sample size was 365, resulting in a power of 99.8%, which provides high confidence in our results. In terms of gestational age, the NEC group included infants with gestational ages ranging from 28 to 36 weeks, while the control group included infants with gestational ages ranging from 28.3 to 36.6 weeks. The NEC group had an average gestational age of 32.5 ± 2.9 weeks, while the control group had an average gestational age of 33.4 ± 2.3 weeks, demonstrating a significant difference between the two groups (P = 0.003). Regarding birth weight, the NEC group had an average birth weight of 2040 ± 206 g, whereas the control group had an average birth weight of 2120 ± 213 g; these values also exhibited a significant difference (P = 0.001). The antenatal steroid use was similar in both groups (P = 0.702). There was no substantial disparity in the sex ratio between the two groups (P = 0.529). Furthermore, there was no notable variation in feeding characteristics, whether breastfeeding, formula feeding, or both—between the two groups (P > 0.050, Table I). The onset of NEC in preterm infants was about two weeks after birth.
| NEC, necrotizing enterocolitis; SD, standard deviation. | |||
| Table I. The clinical information of two study groups. | |||
| Characteristics |
|
|
|
| Gestational age, weeks, mean±SD |
|
|
|
| Birth weight, grams, mean±SD |
|
|
|
| Antenatal steroid use, n (%) |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Sex, n (%) |
|
||
| Male |
|
|
|
| Female |
|
|
|
| Feeding type, n (%) |
|
||
| Breastmilk |
|
|
|
| Formula |
|
|
|
| Both |
|
|
|
| Time of diagnosis, week, mean±SD |
|
||
The genotype and allele frequencies of miR-149 rs2292832 gene polymorphisms
The distribution of allele and genotype frequencies of the miR-149 rs2292832 polymorphism in NEC patients is summarized in Table II. Analysis of the rs2292832 genotype revealed a significant association between the T allele and NEC onset, with a high occurrence frequency of 77.45% (χ2 = 10.527, 95% CI = 1.848 [1.271-2.686], P = 0.001). Conversely, the C allele showed a relatively lower association, occurring at a frequency of only 22.55%. Within the NEC group, there was a relatively higher frequency of homozygous TT genotype (62.75%) than the control group (45.25%), while heterozygous mutant genotype CT and recessive homozygous CC genotypes had lower frequencies in this group (29.41% and 7.84%, respectively) than the control group (39.54% and 15.21%, respectively). In summary, the T allele of rs2292832 genotype was associated with an increased risk for NEC susceptibility compared to the relatively low genetic predisposition observed with the C allele (Table II).
| CI, confidence interval; NEC, necrotizing enterocolitis; OR, odds ratio. | |||||
| Table II. The genotype and allele frequencies of miR-149 rs2292832 gene polymorphisms. | |||||
| Genotype / Allele |
|
|
|
|
|
| Genotype | |||||
| CC |
|
|
|
|
|
| CT |
|
|
|
|
|
| TT |
|
|
|
|
|
| Allele | |||||
| C |
|
|
|
|
|
| T |
|
|
|
|
|
The rs2292832 locus constituted a significant risk factor for the development of NEC in premature infants
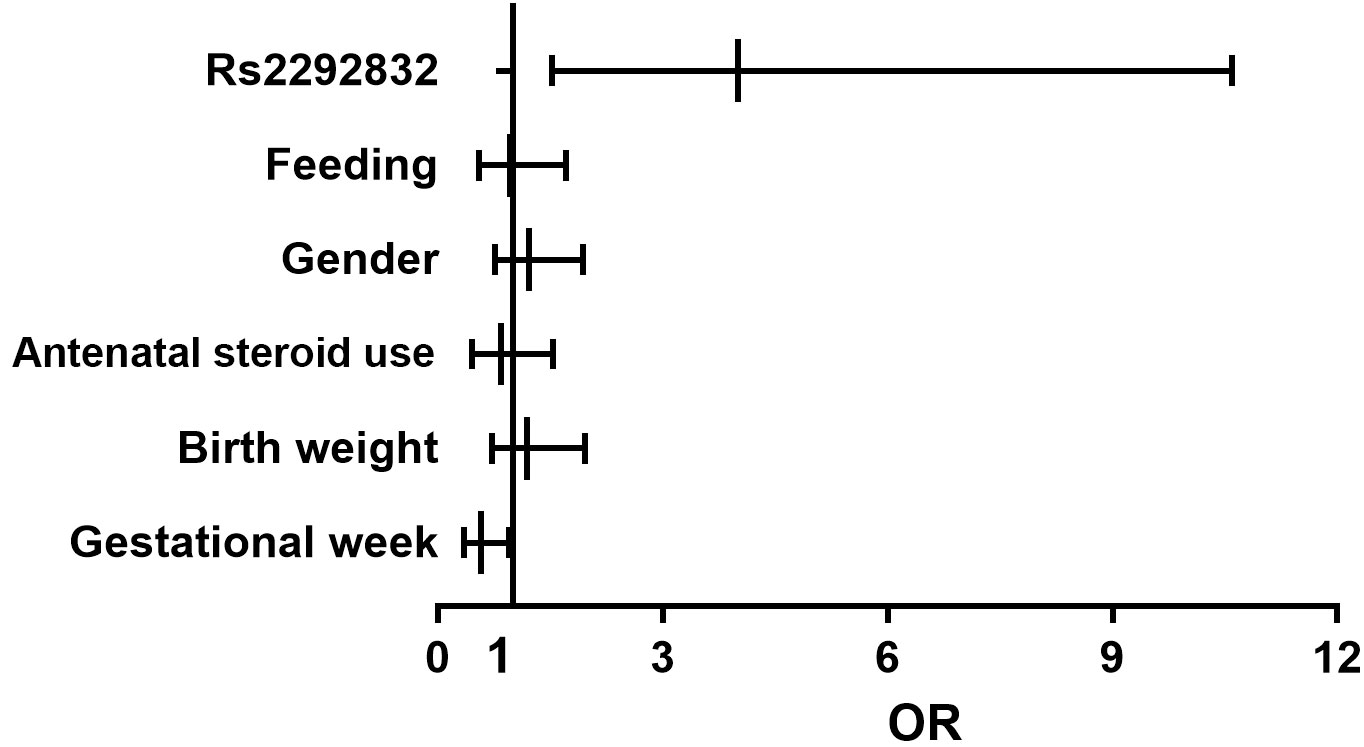
The association between miR-149 rs2292832 and the risk of NEC in premature infants was further investigated using multivariate logistic regression analysis. In this analysis, gestational age showed a significant association with NEC risk (odds ratio [OR] = 0.574, 95% CI 0.348-0.947, P = 0.030. However, birth weight, antenatal steroid use, sex, and feeding did not show a significant association with NEC risk (P > 0.050). On the other hand, the genotype rs2292832 polymorphism exhibited a significantly positive association with NEC risk (OR = 4.009, 95% CI 1.517-10.597, P = 0.005, Table III). The forest plot visually demonstrates that the miR-149 rs2292832 polymorphism was the most prominent risk factor for NEC development in preterm infants (Fig. 1).
| CI, confidence interval; NEC, necrotizing enterocolitis; OR, odds ratio. | |||
| Table III. Multivariate logistic regression analysis of risk factors for NEC in preterm infants. | |||
| Variables |
|
||
|
|
|
|
|
| Gestational age |
|
|
|
| Birth weight |
|
|
|
| Antenatal steroid use |
|
|
|
| Sex |
|
|
|
| Feeding |
|
|
|
| Rs2292832 |
|
|
|
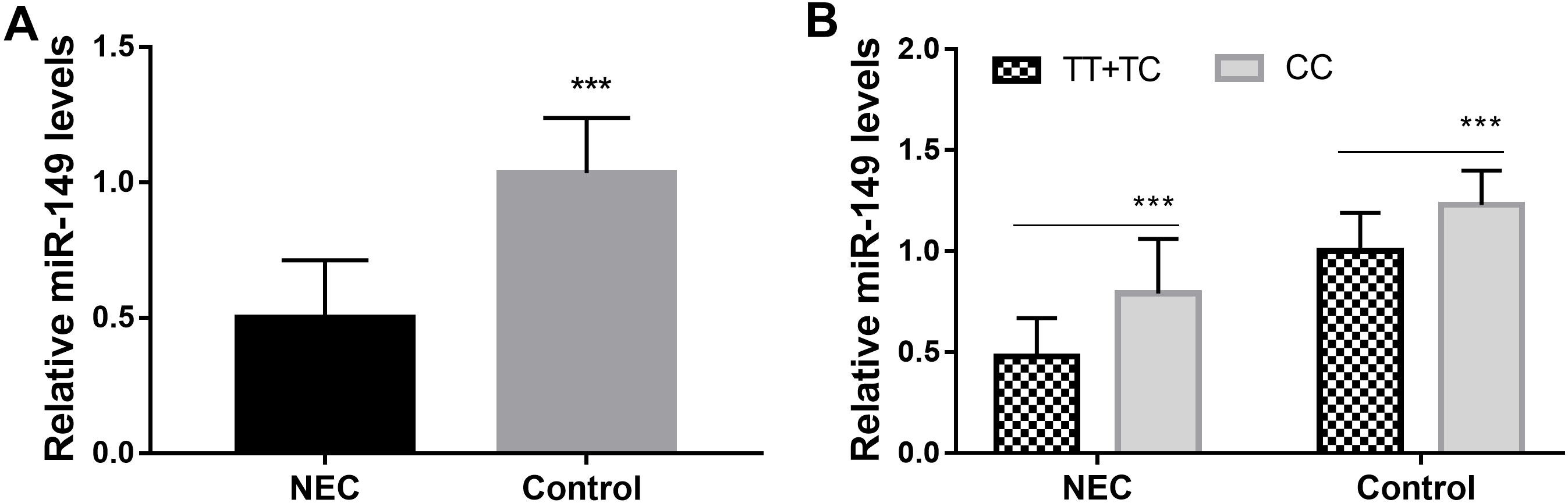
The expression level of miR-149 was downregulated in individuals with NEC
RT-qPCR analysis was employed to assess miR-149 expression levels in individuals from both groups and revealed that serum miR-149 expression level was significantly reduced in NEC compared to controls (Fig. 2A). To investigate the association between miR-149 gene polymorphism locus rs2292832 and preterm infants with NEC, we further examined expression levels across different genotypes within both the NEC and control groups. The results demonstrated that within both groups, the homozygous CC genotype exhibited significantly higher miR-149 expression levels than the mutant TT + TC genotype did; moreover, this gap in miR-149 expression level between CC genotype and mutant TT + TC genotype appeared more pronounced within the NEC group compared to controls (Fig. 2B). These findings suggested the potential association between miR-149 gene polymorphism locus rs2292832 and NEC might be related to its mediation in miR-149 levels.
Discussion
In this study, the expression level of serum miR-149 was significantly lower in preterm infants with NEC, and the allele frequency of the miR-149 rs2292832 polymorphism differed between the NEC and control groups. Specifically, the T allele and TT genotype of rs2292832 were associated with an increased susceptibility to NEC. NEC is a severe gastrointestinal disorder with significant implications in the neonatal period, often necessitating urgent surgical intervention and posing a grave threat to life.20 Khasawneh et al.21 have highlighted that preterm infants constitute the primary high-risk group for developing NEC. Despite extensive research efforts, the pathogenesis of NEC remains elusive; however, given its variable severity among newborns, genetic factors may play a pivotal role in its onset. Notably, CPS1 T1405N polymorphism may be associated with the risk of NEC in preterm infants22, and functional variants of the CPS1 gene may be associated with NEC susceptibility.23 Our study aimed to investigate polymorphisms associated with NEC and elucidate potential mechanisms underlying this condition to facilitate the development of personalized treatment strategies.
Investigating candidate genes associated with the occurrence and progression of NEC can provide insights into the molecular mechanisms underlying NEC. This study aimed to explore the relationship between a genotype polymorphism (rs2292832) in the miR-149 gene and NEC. The study included 102 preterm infants with NEC and 263 infants without NEC hospitalized during the same period. The analysis of clinical data revealed a significant association between gestational age and birth weight in both the NEC group and the control group, as indicated by our findings. The results revealed significant associations between gestational age, rs2292832 polymorphism, and NEC risk. Furthermore, the miR-149 rs2292832 polymorphism emerged as a prominent risk factor for NEC development in preterm infants. Additionally, serum levels of miR-149 were significantly reduced in children with NEC, consistent with previous research findings, indicating decreased expression of serum miR-149-5p in patients with inflammatory bowel disease.19 In both the NEC group and control group, the homozygous CC genotype exhibited significantly higher expression levels of miR-149 compared to mutant TT + TC genotypes; moreover, this difference was more pronounced within the NEC group than in controls. Previous studies have linked miR-149 TT (T > C, rs2292832) to an increased risk of spontaneous abortion16, while suggesting that the C allele of miR-149 rs2292832 may confer protection against gastric mucosal atrophy.17 In the Chinese Han population, carrying the TT genotype or T allele for rs2292832 polymorphism in the miR-149 gene has been found to elevate gastric cancer risk.24 Similarly, the TT genotype has been associated with the clinical stage of nasopharyngeal carcinoma.25 Moreover, Iranian population studies have shown an association between the TT genotype of rs2292832 in miR-149 and coronary artery disease.26 Additionally, they have found a significant correlation between the recessive genetic model TT / (TC + CC) for mir-RS299832 and cancer susceptibility.18 After analyzing the rs2292832 genotype, it was observed that the allele T exhibits a significant association with the onset of NEC, while the proportion of heterozygous mutant genotype CT and recessive homozygous CC genotype in the NEC group was relatively small. Therefore, it can be inferred that the T allele of rs2292832 genotype was linked to an increased susceptibility to NEC, whereas the C allele demonstrates a comparatively lower association. These findings suggest that rs2292832 genotype polymorphism may be implicated in NEC risk, with individuals carrying the T allele or TT genotype being more susceptible to NEC.
Gene polymorphism can influence gene expression and function. In individuals with the rs2292832 genotype, those carrying the TT genotype demonstrate an increased susceptibility to NEC. This association may be attributed to the downregulation of miR-149 expression mediated by the TT genotype. Additionally, studies have demonstrated decreased expression levels of serum miR-149-5p in patients with inflammatory bowel disease.19 This also coincided with our research findings regarding the lower expression level of miR-149 in NEC individuals. MiR-149 has been extensively reported in the literature for its involvement in various biological processes such as cell proliferation, apoptosis, and inflammatory response.27 Dysregulated miR-149 expression could potentially compromise intestinal mucosal barrier function and exacerbate inflammatory responses, thereby heightening the risk of NEC. These studies provided compelling evidence supporting our conclusions while further emphasizing the intricate relationship between genotype, miR-149, and NEC. It is proposed that miR-149 may play a significant role in the pathogenesis of NEC.
However, this study has several limitations. Despite previous research indicating that breastfeeding confers a protective effect against NEC28, our study did not observe a significant association between feeding methods and the risk of NEC. This discrepancy may be attributed to the relatively smaller sample size, which lacked sufficient statistical power to detect the subtle influence of feeding methods on NEC risk. Although we endeavored to match relevant factors between the control and NEC groups, unexplained variables may still have influenced the results. Additionally, fetuses in the control group were generally more mature than those in the patient group, possibly due to the samples primarily originating from a single hospital with limited capacity for preterm infants. The relatively small sample size of the included standard samples may also contribute significantly to this deviation. This difference underscores the higher likelihood of NEC development in preterm infants with smaller gestational ages. Moreover, this study focused on the relationship between the miR-149 gene rs2292832 polymorphism and NEC susceptibility but did not analyze genetic variations associated with different stages of NEC or provide data on other inflammatory issues, intestinal perforation, or patient prognosis. Future studies should expand the sample size to explore genetic differences in these areas and provide more evidence for accurate diagnosis and treatment of NEC in preterm infants.
In conclusion, miR-149 rs2292832 polymorphism may be implicated in NEC. Specifically, the T allele of rs2292832 exhibited a significant correlation with NEC morbidity, and within NEC patients, there was a relatively high prevalence of individuals carrying the T allele and TT genotype.
Ethical approval
This study has been approved by the Women and Children’s Hospital of Ningbo University Medical Ethics Committee. All procedures adhere to the principles outlined in the Helsinki Declaration. Informed consent forms have been signed by all newborn guardians.
Source of funding
This study was funded by the Project of Ningbo Leading Medical & Health Discipline, China (No. 2010-S04).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Kordasz M, Racine M, Szavay P, et al. Risk factors for mortality in preterm infants with necrotizing enterocolitis: a retrospective multicenter analysis. Eur J Pediatr 2022; 181: 933-939. https://doi.org/10.1007/s00431-021-04266-x
- Campos-Martinez AM, Expósito-Herrera J, Gonzalez-Bolívar M, Fernández-Marin E, Uberos J. Evaluation of risk and preventive factors for necrotizing enterocolitis in premature newborns. A systematic review of the literature. Front Pediatr 2022; 10: 874976. https://doi.org/10.3389/fped.2022.874976
- Zozaya C, García González I, Avila-Alvarez A, et al. Incidence, treatment, and outcome trends of necrotizing enterocolitis in preterm infants: a multicenter cohort study. Front Pediatr 2020; 8: 188. https://doi.org/10.3389/fped.2020.00188
- Zhang Y, Ma JK, Wei H, Li XW, Li LQ, Yu JL. Predictive scores for mortality in full-term infants with necrotizing enterocolitis: experience of a tertiary hospital in Southwest China. World J Pediatr 2016; 12: 202-208. https://doi.org/10.1007/s12519-015-0063-x
- Alganabi M, Lee C, Bindi E, Li B, Pierro A. Recent advances in understanding necrotizing enterocolitis. F1000Research 2019; 8(F1000 Faculty Rev): 107. https://doi.org/10.12688/f1000research.17228.1
- Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med 2011; 16: 145-150. https://doi.org/10.1016/j.siny.2011.02.002
- Bethell GS, Hall NJ. Recent advances in our understanding of NEC diagnosis, prognosis and surgical approach. Front Pediatr 2023; 11: 1229850. https://doi.org/10.3389/fped.2023.1229850
- Cuna A, George L, Sampath V. Genetic predisposition to necrotizing enterocolitis in premature infants: current knowledge, challenges, and future directions. Semin Fetal Neonatal Med 2018; 23: 387-393. https://doi.org/10.1016/j.siny.2018.08.006
- Sun G, Yan J, Noltner K, et al. SNPs in human miRNA genes affect biogenesis and function. RNA 2009; 15: 1640-1651. https://doi.org/10.1261/rna.1560209
- Guo L, Tsai SQ, Hardison NE, et al. Differentially expressed microRNAs and affected biological pathways revealed by modulated modularity clustering (MMC) analysis of human preeclamptic and IUGR placentas. Placenta 2013; 34: 599-605. https://doi.org/10.1016/j.placenta.2013.04.007
- Zhao X, Wang Y, Li L, Mei J, Zhang X, Wu Z. Predictive value of 4-Hydroxyglutamate and miR-149-5p on eclampsia. Exp Mol Pathol 2021; 119: 104618. https://doi.org/10.1016/j.yexmp.2021.104618
- Gao Y, Guo X, Li Y, Sha W, She R. The decreased lncRNA ZEB2-AS1 in pre-eclampsia controls the trophoblastic cell line HTR-8/SVneo’s invasive and migratory abilities via the miR-149/PGF axis. J Cell Biochem 2019; 120: 17677-17686. https://doi.org/10.1002/jcb.29034
- Hu Z, Dong C, Dong Q. Circ_0015382 is associated with preeclampsia and regulates biological behaviors of trophoblast cells through miR-149-5p/TFPI2 axis. Placenta 2021; 108: 73-80. https://doi.org/10.1016/j.placenta.2021.03.005
- Liu R, Wang X, Yan Q. The regulatory network of lncRNA DLX6-AS1/miR-149-5p/ERP44 is possibly related to the progression of preeclampsia. Placenta 2020; 93: 34-42. https://doi.org/10.1016/j.placenta.2020.02.001
- Wang X, Xu Q, Wang S. Overexpression of miR-149-5p attenuates cerebral ischemia/reperfusion (I/R) injury by targeting Notch2. Neuromolecular Med 2022; 24: 279-289. https://doi.org/10.1007/s12017-021-08685-9
- Jeon YJ, Kim SY, Rah H, et al. Association of the miR-146aC>G, miR-149T>C, miR-196a2T>C, and miR-499A>G polymorphisms with risk of spontaneously aborted fetuses. Am J Reprod Immunol 2012; 68: 408-417. https://doi.org/10.1111/aji.12005
- Qi L, Chang YU, Jintong YE, et al. Association of miR-499 rs3746444, miR-149 rs2292832 polymorphisms and their expression levels with helicobacter pylori-related gastric diseases and traditional Chinese medicine syndromes. J Tradit Chin Med 2024; 44: 1024-1034. https://doi.org/10.19852/j.cnki.jtcm.2024.05.009
- Alidoust M, Hamzehzadeh L, Rivandi M, Pasdar A. Polymorphisms in non-coding RNAs and risk of colorectal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2018; 132: 100-110. https://doi.org/10.1016/j.critrevonc.2018.09.003
- Luo S, Chen XH. Tissue and serum miR-149-3p/5p in hospitalized patients with inflammatory bowel disease: correlation with disease severity and inflammatory markers. Kaohsiung J Med Sci 2024; 40: 131-138. https://doi.org/10.1002/kjm2.12784
- Wertheimer F, Arcinue R, Niklas V. Necrotizing enterocolitis: enhancing awareness for the general practitioner. Pediatr Rev 2019; 40: 517-527. https://doi.org/10.1542/pir.2017-0338
- Khasawneh W, Khriesat W. Assessment and comparison of mortality and short-term outcomes among premature infants before and after 32-week gestation: a cross-sectional analysis. Ann Med Surg (Lond) 2020; 60: 44-49. https://doi.org/10.1016/j.amsu.2020.10.017
- Moonen RM, Paulussen AD, Souren NY, Kessels AG, Rubio-Gozalbo ME, Villamor E. Carbamoyl phosphate synthetase polymorphisms as a risk factor for necrotizing enterocolitis. Pediatr Res 2007; 62: 188-190. https://doi.org/10.1203/PDR.0b013e3180a0324e
- Moonen RM, Cavallaro G, Huizing MJ, González-Luis GE, Mosca F, Villamor E. Association between the p.Thr1406Asn polymorphism of the carbamoyl-phosphate synthetase 1 gene and necrotizing enterocolitis: a prospective multicenter study. Sci Rep 2016; 6: 36999. https://doi.org/10.1038/srep36999
- Zhang L, Liu Q, Wang F. Association between miR-149 gene rs2292832 polymorphism and risk of gastric cancer. Arch Med Res 2018; 49: 270-277. https://doi.org/10.1016/j.arcmed.2018.09.012
- Huang GL, Lu Y, Pu XX, et al. Association study between miR-149 gene polymorphism and nasopharyngeal carcinoma. Biomed Rep 2013; 1: 599-603. https://doi.org/10.3892/br.2013.97
- Ghaffarzadeh M, Ghaedi H, Alipoor B, et al. Association of MiR-149 (RS2292832) variant with the risk of coronary artery disease. J Med Biochem 2017; 36: 251-258. https://doi.org/10.1515/jomb-2017-0005
- Hu W, Jiang Y, Wen C, Zeng Y, Jia M. MiR-149-5p inhibits cell proliferation, promotes cell apoptosis and retards cell cycle of IL-22-stimulated HaCaT and NHEK keratinocytes via regulating PDE4D. Cytokine 2023; 164: 156123. https://doi.org/10.1016/j.cyto.2023.156123
- Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010; 156: 562-567. https://doi.org/10.1016/j.jpeds.2009.10.040
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.