Abstract
Animal allergens, particularly those from cats, dogs, and horses, are significant risk factors for the development of allergic diseases in childhood. Managing animal allergies requires allergen avoidance and, when this is not feasible, specific immunotherapy. Patient history remains the cornerstone of diagnosis, providing the foundation for diagnostic algorithms. Extract-based tests, such as skin prick tests and specific IgE measurements, are essential for confirmation and screening. However, traditional extract-based diagnostic methods have notable limitations, as they are unable to distinguish between primary sensitization and immunological cross-sensitization, and also has the potential for both false negatives and false positives. Polysensitization may arise from either multiple independent sensitizations (co-sensitization) or cross-sensitizations, between homologous allergens. Due to complex cross-reactivity and polysensitization in mammals, extract-based tests are often insufficient in determining the true allergen, so molecular allergen testing should be used. Even with molecular testing, there is no consensus on how to define complex and intriguing sensitization patterns in mammals. In this report, we review the literature on cat, dog, and horse allergies and propose a novel approach to identifying complex sensitization patterns based on the current state of knowledge. We recommend that the evaluation of cat, dog, and horse allergies should begin with investigating genuine sensitization to Fel d 1, Can f 4/5, and Equ c 4, respectively. As a subsequent step, we propose a practical approach to determine primary allergen sensitization within the lipocalin group. Secondary sensitizations should then be evaluated in the context of recent contact history and presenting symptoms. While serum albumin is less strongly associated with true animal allergies, we suggest that it may serve as a complementary marker when considered alongside cross-reactive food allergen molecules.
Keywords: allergy, allergen molecule, cat, dog, horse, sensitization
Exposure to Animal Allergens
Humans have coexisted with cats, dogs, and horses since the dawn of civilization. While their traditional roles in pest control, protection, and transportation have diminished with technological advancements, modern life has not reduced our contact with these animals. On the contrary, they have become increasingly integrated into our homes and daily lives. Developing with pets can contribute to a child’s self-esteem and confidence as well as developing non-verbal communication and empathy. Pet ownership rates vary in different parts of the world due to regional and cultural differences. Up to 50% of European households own a pet. Although the number of pet owners is one of the strongest predictors of increased allergen levels, high allergen levels have also been found in places where there are no pets (e.g. schools and public places). Animal allergens can become ubiquitous through passive transfer via human clothing or hair spread by pet owners.1 In addition, there are significant stray animal populations in many parts of the world, including the Eastern Mediterranean region. Stray animals shed allergens (e.g. hair, dander, saliva and urine) into the environment, resulting in widespread exposure in public and residential areas. Stray animals, in lesser quantities, contribute to widespread environmental exposure to allergens. Although adoption rates are low (5% for dogs and 14% for cats), the high number of stray animals in Turkey indicates a high level of exposure.2
Prevalence of Sensitization to Cats, Dogs and Horses
Although there are regional differences in sensitization to furry animals, there has been an increase in sensitization in recent years. Sensitization to cats and dogs was found in 26% and 27% of adults in Europe, respectively.3 In the United States, the prevalence of cat sensitization in subjects aged 6 years and older was 12.1%, whereas the incidence of dog sensitization was 11.8%.4
Horse sensitization is expected to be higher in those who have close contact with horses for professional or recreational purposes. In one study, horse sensitization was found to be 4.3% in the general population compared to 12.8% in grooms.5 However, indirect exposure is also important in the equine sensitization. Another study found that 5.4% of patients were sensitized to horses, although most of the patients did not have contact with them. There are several possible routes of exposure to equine allergens, including airborne dispersion and indirect exposure through clothing.6
The prevalence of sensitization is modified by the age of the subject, with an increase during childhood and adolescence.7 However, it should be emphasized that prevalence studies were carried out using extract-based tests. If the extract’s constituents or the strength changes over time, this could lead to an apparent difference in prevalence.8
Impact of Pet Allergens on Allergic Diseases
Allergens from cats, dogs and horses are major risk factors for the development of asthma and allergic rhinitis. Sensitization to some allergen molecules and higher levels of IgE to allergen molecules are associated with an increased risk of allergic diseases. For example, asthmatic children with cat allergy have higher Fel d 1 sIgE levels than children with rhinitis alone.9 One study reported that sensitization to Can f 2 and Equ c 1 was more common in severe asthma. IgE levels to Equ c 1 -a horse allergen molecule- correlated with asthma control. Children with higher levels of IgE antibodies to cats, dogs and horses had severe asthma and more bronchial hyperresponsiveness.10 Sensitization to furry animal allergen molecules is an important predictor of asthma outcome and an indicator of severity.11 It was reported that sensitization to more components was associated with increased airway inflammation.11,12 One study reported that multi-sensitization towards lipocalin, kallikrein and secretoglobin molecules was more common among severe asthmatics compared to children with controlled asthma. These subjects also had higher blood eosinophils, higher fractional exhaled nitric oxide and increased bronchial hyperresponsiveness.13
Treatment is based on identifying the allergens causing symptoms and counseling patients to avoid these allergens. Reducing allergen exposure often requires removing the cat or dog from the home, which underscores the importance of accurately identifying the true allergen causing clinical symptoms. However, in most cases, removing the animal is not feasible due to the emotional bond between the animal and the child, as well as the parents. In such cases, specific immunotherapy is a valid option, but it is critical to identify children who are most likely to benefit from this therapy. Due to complex cross-reactivity and polysensitization among mammalian allergens, determining the true allergen can often be challenging.8,14 In recent years, new strategies have been developed to neutralize Fel d1 at its source. Since the biological function of Fel d 1 in cats is currently unknown, studies have focused on developing approaches that neutralize Fel d 1 after it is produced, without altering its natural production. Satyaraj et al showed that feeding cats a diet with an egg product ingredient containing anti-Fel d 1 IgY reduces environmental Fel d 1 levels and produces a significant improvement in the nasal and ocular symptoms cat-allergic humans.15
Molecular Allergy Diagnostics for Animal Allergies
Patient history remains the cornerstone of diagnosis where diagnostic algorithms converge. Extract-based tests, such as skin prick tests and specific IgE measurements, are essential for confirmation and screening. Traditional extract-based diagnostics have major limitations. Firstly, there is no standardization of the allergens used as substrates. As a result, the concentration of allergens in extracts varies widely. Extract-based tests may sometimes lack certain allergens or be contaminated with irrelevant ones. This is likely to lead to false negative or false positive results, which could have a negative impact on the accuracy of the diagnosis of animal allergy. For example, patients with a history of allergy but no evidence of sensitization on extract-based tests may require molecular testing.14,16,17
One of the major diagnostic challenges in animal allergy is that in routine testing, up to 75% of subjects sensitized to animal dander are also sensitized to two or more different species.18 Conventional allergy testing with whole extracts can detect polysensitization well but cannot differentiate between primary sensitization and immunological cross-reactivity. Polysensitization can be based on multiple independent sensitizations (co-sensitization) or cross-sensitizations between homologous allergens. This can lead to false positive results. Patients who are found sensitized to extract-based tests but have no clear clinical symptoms or history of exposure to the detected allergen require further evaluation. The analysis and assignment of complex sensitization patterns are only possible with the growing availability of recombinant or purified native animal allergens for singleplex and multiplex testing. Therefore, in most cases, molecular allergology evaluation is necessary.16,17
In addition, component resolved diagnostic tests provide insight into the severity and outcome of asthma and allergic rhinitis.11 Nwaru et al investigated 1872 adults for cat, dog and horse allergen serum IgE levels. They used cluster analysis to derive distinct sensitization clusters and show their association with asthma, rhinitis and markers of asthma severity in adults. Sensitization to furry animal allergen components is an important predictor of asthma, rhinitis, and markers of asthma severity with increased blood eosinophils, fractional exhaled nitric oxide, and airway hyperreactivity.11
Allergen molecule sensitizations measured by singleplex arrays are generally preferred to multiplex arrays due to their ability to provide precise, quantitative sensitization data, although they are limited by the number of available animal allergens. While multiplex platforms offer several advantages, including a comprehensive view of sensitizations, reduced serum requirements, and cost-effectiveness when testing for more than 12 allergens, they also have drawbacks, such as lower analytical sensitivity-particularly at low total IgE levels-semiquantitative sensitization data, and potential interference from IgG.19,20
Animal Allergen Molecules
The cat, dog, and horse allergen molecules identified to date, along with their respective groups, are listed in Table I. Fel d 1 is a glycoprotein belonging to the secretoglobin family. Fel d 1 is a thermostable protein that is found in the saliva, anal glands, sebaceous glands, skin and fur of cats. Fel d 1 spreads easily. Not all cats shed Fel d 1 at the same rate, hormonal status modifies its production. For example, males have been shown to produce more Fel d 1 than females. In addition, neutered males produce less Fel d 1 than unneutered males.21 Fel d 1 is a major cat allergen. Fel d 1-specific IgE has been shown to be as reliable as IgE to cat extract for the diagnosis of cat allergy.9,22
| Table I. Cat, dog and horse allergen molecules according to Allergen Nomenclature by WHO/IUIS Allergen Nomenclature Sub-Committee (https://www.allergen.org). | |
| Allergen molecule | Biochemical name |
| Cat | |
| Fel d 1 | Secretoglobin |
| Fel d 2 | Serum albumin |
| Fel d 3 | Cystatin-A |
| Fel d 4 | Lipocalin |
| Fel d 5 | Immunoglobulin A |
| Fel d 6 | Immunoglobulin M |
| Fel d 7 | Lipocalin |
| Fel d 8 | Latherin |
| Dog | |
| Can f 1 | Lipocalin |
| Can f 2 | Lipocalin |
| Can f 3 | Serum albumin |
| Can f 4 | Lipocalin |
| Can f 5 | Kallikrein |
| Can f 6 | Lipocalin |
| Can f 7 | Niemann Pick type C2 |
| Can f 8 | Cystatin |
| Horse | |
| Equ c 1 | Lipocalin |
| Equ c 2 | Lipocalin |
| Equ c 3 | Serum albumin |
| Equ c 4 | Latherin |
| Equ c 6 | Lysozyme |
Can f 4 is a major dog allergen from the lipocalin family. It is associated with true dog allergy which has been demonstrated in some studies.8 In a recent study23, it was reported that sensitizations to Can f 4 indicates genuine sensitizations to dogs.
Can f 5 has been identified as the major dog allergen, a prostatic kallikrein expressed in the prostate gland and is consequently only present in male dogs. In addition, castration of male dogs has been shown to drastically reduce its production. Can f 5 is mainly found in the urine of male dogs, but also in extracts of dog hair and dander. Direct contact with the male dog is important for sensitization as it does not spread as easily as lipocalins.24 Recently, Schoos et al. showed that patients allergic to male dogs and monosensitized to Can f 5 tolerated a conjunctival challenge with female dog extract.25
Horses produce latherin, a highly surface active, non-glycosylated protein. Latherin was detected in horse skin and salivary glands.26 Up to 77% of patients with horse allergy are sensitized to Equ c 4, a latherin protein.27 Equ c 4 may be a specific marker for horse allergy but this needs to be further evaluated.8 In a recent study23 it was reported that sensitizations to Equ c 4 differed from other horse allergen molecule sensitizations, indicating genuine sensitizations to horses.
Fel d 1, a secretoglobin protein, is the primary cause of IgE-mediated reactions in cat-allergic individuals. While dogs do not produce Fel d 1, a structurally similar protein in dog hair extracts has been identified, which may explain dual sensitization to both cat and dog allergens.8 In a pediatric study23, sensitization to the Fel d 1-like protein was observed in only a subset of Fel d 1-sensitized individuals (38 of 95), with more than 50% of these (22 of 38) not sensitized to other dog allergens. Quantitative analysis confirmed that Fel d 1 sensitization predominated in all dually sensitized individuals. The authors suggested that the Fel d 1-like protein in dogs likely results from cross-sensitization to Fel d 1 in cats. The clinical significance of Fel d 1-like protein sensitization warrants further investigation to elucidate its specific role in dog allergy.16,23,28
Lipocalins are the most important group of inhaled animal allergens. They are small, secreted molecules that are easily spread in indoor environments. They are produced in the secretory glands or liver and are found in saliva, urine and hair dander. They share a characteristic tertiary structure with a central β-barrel formed by 8 anti-parallel β-strands. Lipocalin sequence identities between family members can be as low as 15%, while some lipocalins have much higher sequence identities, up to 67%. Due to pairs with high sequence identity, lipocalins may contribute to allergic cross-sensitizations between different species. Fel d 4 and Fel d 7 in cats, Can f 1, Can f 2, and Can f 6 in dogs, Equ c 1 in horses are allergen molecules belonging to the lipocalin family with cross-sensitization potential.8,29
Serum albumins are mainly respiratory allergens, but sensitization to serum albumins may be more complex and involve multiple routes of exposure. They are abundant in blood, but are also present in milk, saliva, dander and meat. It is highly likely that contact with aminal dander is the major source of health problems associated with serum albumin in humans. Serum albumins are considered minor allergens. Due to their high sequence similarity (up to 70%), cross-sensitization is one of the most important features of these allergens. In many cases, individuals allergic to serum albumin are polysensitized to different animals. Serum albumins are thermolabile proteins and their allergenicity is inactivated by heat. Bos d 6 in cattle, Can f 3 in dogs, Equ c 3 in horses, Fel d 2 in cats, Sus s 1 in pigs are examples of mammalian serum albumin allergens.8,30
There are other allergen molecules that belong to other allergen families in animals. For example, Fel d 3 and Can f 8 belong to the cystatin A protein family. Recently, Niemann-Pick type C proteins have been identified in both cats and dogs.8
Co-sensitization has to be distinguished from cross-sensitization. It is important to recognize that IgE-cross-sensitization may not always imply clinical cross-reactivity. When cross-sensitized lipocalin allergens have high sequence homology, patients may experience symptoms of all these allergen sources. Certain lipocalins (Can f 6, Fel d 4, Equ c 1; Fel d 7 and Can f 1) share a high sequence identity and serve as markers of cross-sensitization. However, there is limited data on symptoms clearly related to cross-sensitized molecules as monosensitization to these allergen molecules seems to be rare.8,16
Many animal allergens are pan-allergens, and most lipocalins and serum albumins exhibit complex cross-sensitization patterns, contributing significantly to polysensitization. However, the interpretation of sensitizations in the diagnosis of cat, dog, and horse allergies remains a topic of debate. While there is general consensus among experts and guidelines on the diagnostic approach, inconsistencies remain in the recommendations.8,31-34
Regarding cat allergy, there is a consensus that Fel d 1 is the genuine allergen, and in most cases, detecting sensitization to Fel d 1 is sufficient for diagnosis. Typically, sensitization to Fel d 4 and/or Fel d 7 develops after Fel d 1 sensitization8,23 a phenomenon known as molecular spreading.35 However, in rare cases, sensitization to cat lipocalins may precede Fel d 1 sensitization. Current diagnostic algorithms do not clearly define when to consider cat allergy in the absence of Fel d 1 sensitization, even if sensitization to Fel d 4 and/or Fel d 7 is present, which cross-reacts with Equ c 1 and Can f 1, respectively.14,16,23,31,33,36,37
For dog allergy, guidelines consistently recognize Can f 5 as a marker of genuine sensitization to male dogs and Can f 1/6 as major contributors to dog sensitization.8,14,16,23,31,34,36-39 While Can f 1 and/or Can f 6 sensitizations may indicate dog allergy in the absence of other lipocalin sensitizations, their association with dog allergy is inconsistent when other lipocalin sensitizations are present. Additionally, there is no consensus on which allergen molecule sensitizations should be investigated in the absence of Can f 5 sensitization. Given the high cross-sensitization within the animal lipocalin group, it may be logical to evaluate all furry animal lipocalins (Fel d 4/7, Can f 1/2/6, Equ c 1) simultaneously to identify the primary sensitizer.
Regarding horse allergy, diagnostic algorithms consistently identify Equ c 1 as the major allergen.8,27 Recent findings suggest that Equ c 4 may be a genuine sensitizer for horses23 representing genuine sensitization that has not been widely recognized to date. Additionally, Equ c 3, a serum albumin, has limited information regarding its association with horse allergy. While serum albumins are known to exhibit high cross-reactivity among different animal species (Fel d 2, Can f 3, Equ c 3)8, the extent to which sensitization to Equ c 3 alone indicates a related animal allergy and the possibility of cross-sensitization with foods, particularly in children (Sus s 1, Bos d 5), has not been sufficiently studied.
These findings suggest that all furry animal lipocalin and serum albumin sensitizations should be evaluated simultaneously. Therefore, the diagnostic algorithm should not be restricted to a single animal allergy but should encompass a comprehensive evaluation of cross-reactive furry animals, including cats, dogs, and horses.
Further studies are needed to better understand the complex cross-sensitization properties utilizing multiplex assays and to accurately demonstrate patterns of polysensitization and disease-specific sensitization patterns.16 With this aim, in a recent study23 conducted a comprehensive analysis of animal allergen molecules using correlation and hierarchical clustering techniques to elucidate the relationship between cat, dog and horse allergen molecules and to detect primary sensitization, cross-sensitization and co-sensitization. They suggested that a unified algorithm should be used to accurately assess cat, dog and horse allergy, prioritising identification of genuine allergen sensitization first and then elucidating lipocalin sensitization patterns. It was stated that sensitization to Fel d 1, Can f 4/5 and Equ c 4 indicated genuine allergen sensitization for cat, dog and horse allergy, respectively. Although serum albumin is less associated with genuine animal allergy, it has been suggested that it may serve as a complementary marker together with cross-reactive food allergen molecules. They suggested that focusing on the lipocalin and serum albumin groups followed by the assessment of allergy history in the assessment of primary sensitization.23 One of the most critical challenges is to identify the primary sensitizing allergen. A practical and effective approach involves determining the most reactive allergen molecule within the allergen group based on quantitative levels, while considering the coefficient of variation of the measurement method.31,40,41 Identification of the primary sensitizer strongly indicates an allergy to the specific animal in question, but it can also lead to secondary sensitizations due to molecular structural similarities. These secondary sensitizations may or may not represent a true allergic response like the primary sensitizer; therefore, it is essential to carefully consider exposure histories to the relevant animal. Given the need to examine a broad panel of allergen molecules to identify the primary sensitizer within the lipocalin and serum albumin groups, multiplex microarrays offer significant advantages, including cost-effectiveness and reduced serum requirements. However, it should be noted that multiplex arrays provide semiquantitative data and are not as precise as singleplex assays.19
A New Algorithm is Required
Our proposed algorithm differs from previous ones in five key aspects (Fig. 1). First, we suggest a combined top-down and bottom-up approach. The bottom-up approach involves confirming allergies through extract-based assays in patients with a suggestive history of animal allergy. Additionally, the top-down approach emphasizes molecular allergology evaluation in most patients for a more precise diagnosis, particularly in patients with a clinical history but no sensitization through extract-based assays, in patients with sensitization but no close contact or allergy history, in patients requiring immunotherapy, and in patients needing recommendations regarding avoidance or keeping the animal. Second, it identifies Can f 4 and Equ c 4 as additional genuine sensitizers, alongside Fel d 1 and Can f 5. Third, it assumes that genuine and primary sensitizations are the underlying causes of the relevant animal allergies. Fourth, it evaluates cat, dog, and horse allergies within a unified framework, recognizing the presence of numerous allergen molecules with a high likelihood of cross-sensitization. Fifth, it emphasizes the use of multiplex platforms, despite their limitations, due to the necessity of assessing sensitizations to a large number of allergen molecules. However, further studies may be needed to validate and enhance the utility of this algorithm. Its limitations include the exclusion of certain allergen sensitizations, reliance on assumptions not supported by clinical data but rather on hierarchical clustering of sensitizations, and the prioritization of primary sensitization based on quantitative levels, which may carry the potential risk of overdiagnosis.
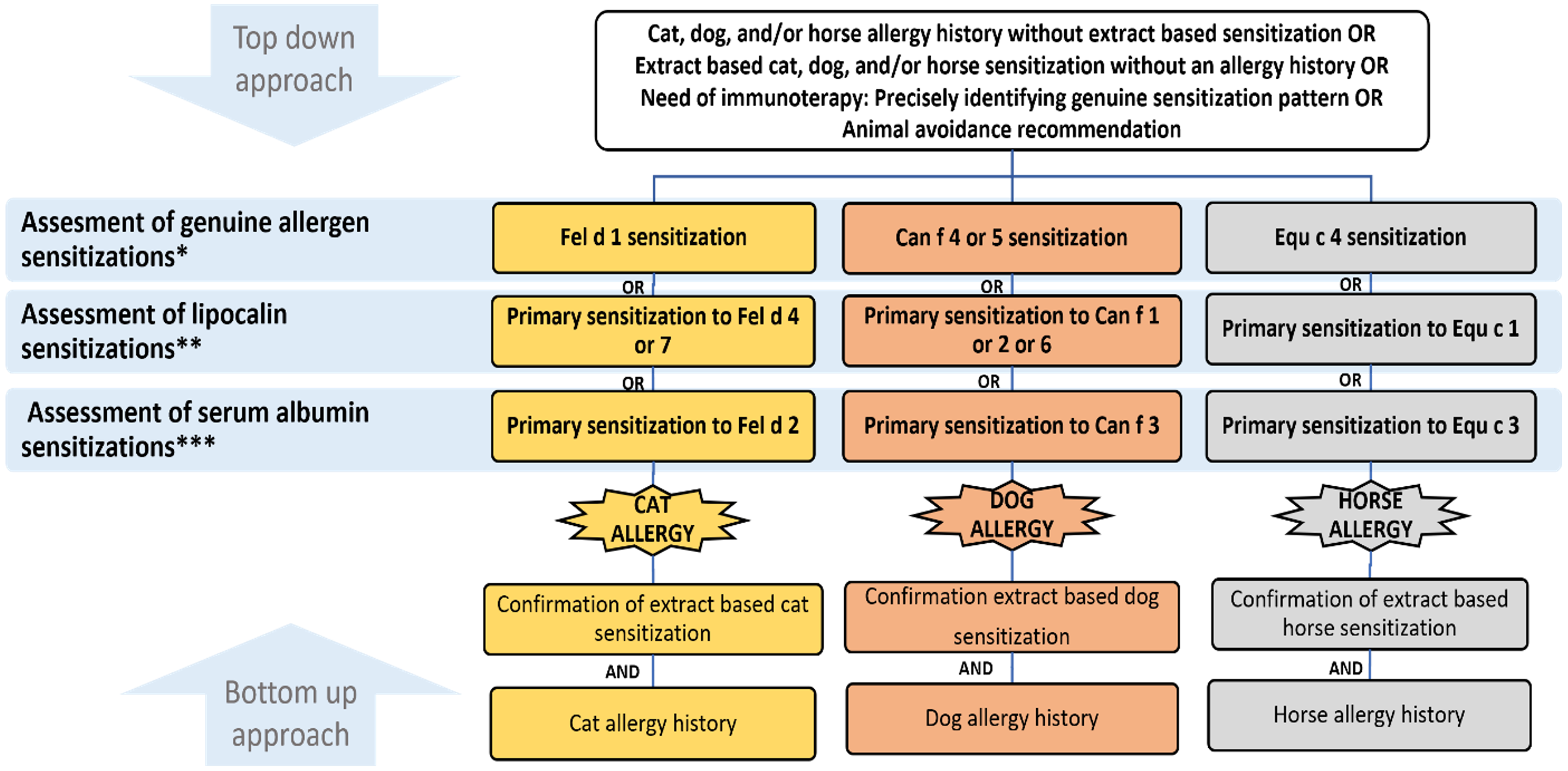
**: The primary sensitization is defined as any allergen with the highest quantitative sensitization within the lipocalin group (Fel d 4/7, Can f 1/2/6, and Equ c 1).
***: The primary sensitization is defined as any allergen with the highest quantitative sensitization within the serum albumin group (Fel d 2, Can f 3, Equ c 3, Bos d 6, Sus s 1)
Management of Cat, Dog and Horse Allergy
As with any allergic condition, the best course of action would be to avoid the offending animal completely. However, this has emotional consequences and is often not possible. There are also animal allergens in environments where animals are not present. There are some measures that focus on reducing exposure to allergens while keeping contact with the animal. These measures are less effective but may be more practical. Regular washing of the pet, keeping the pet out of the bedroom, air cleaning with HEPA filters, regular use and maintenance of high efficiency vacuum cleaners, application of topical lotions to the animal’s fur and a combination of these measures. However, the measures described do not ensure clinical benefit. The effects of neutering and spaying dogs and cats have been inconsistent, and no specific recommendations have been made in this regard.42
Allergen immunotherapy (AIT) is emerging as a potential alternative treatment. However, there is no consensus in the literature regarding cat, dog or horse AIT. Evidence on the efficacy and safety of cat AIT is limited, with no high-quality data on its cost-effectiveness. Some patients, particularly those with moderate-to-severe disease inadequately controlled by allergen avoidance and pharmacotherapy, or those monosensitized to Fel d 1, may benefit from this treatment modality.43 The medical literature on dog extract immunotherapy shows inconsistent and conflicting results regarding clinical efficacy. These outcomes have been attributed to the poor-quality of extracts and the inherently complex profile of dog allergens.44 Similarly, evidence on the safety and efficacy of horse immunotherapy is scarce. AIT with horse extract is not supported by experts, and no consensus has been reached regarding its benefits.27
Conclusion
Animal allergens, particularly from cats, dogs, and horses, present significant diagnostic challenges due to complex cross-sensitization and polysensitization patterns. A stepwise approach, beginning with the identification of genuine sensitization and followed by the determination of the primary sensitizer through molecular allergen testing using multiplex microarrays, can enhance diagnostic accuracy and guide effective management strategies.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Zahradnik E, Raulf M. Animal allergens and their presence in the environment. Front Immunol 2014; 5: 76. https://doi.org/10.3389/fimmu.2014.00076
- Dierks Z. Cat and dog owning households in Turkey 2012-2022. 2023. Available at: https://www.statista.com/statistics/517048/households-owning-cats-dogs-europe-turkey/
- Burbach GJ, Heinzerling LM, Edenharter G, et al. GA(2)LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy 2009; 64: 1507-1515. https://doi.org/10.1111/j.1398-9995.2009.02089.x
- Salo PM, Arbes SJ, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134: 350-359. https://doi.org/10.1016/j.jaci.2013.12.1071
- Tutluoglu B, Atiş S, Anakkaya AN, et al. Sensitization to horse hair, symptoms and lung function in grooms. Clin Exp Allergy 2002; 32: 1170-1173. https://doi.org/10.1046/j.1365-2745.2002.01439.x
- Liccardi G, D’Amato G, Antonicelli L, et al. Sensitization to horse allergens in Italy: a multicentre study in urban atopic subjects without occupational exposure. Int Arch Allergy Immunol 2011; 155: 412-417. https://doi.org/10.1159/000321414
- Roberts G, Zhang H, Karmaus W, et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clin Exp Allergy 2012; 42: 1501-1509. https://doi.org/10.1111/j.1365-2222.2012.04074.x
- Dramburg S, Hilger C, Santos AF, et al. EAACI molecular allergology user’s guide 2.0. Pediatr Allergy Immunol 2023; 34(Suppl 28): e13854. https://doi.org/10.1111/pai.13854
- Grönlund H, Adédoyin J, Reininger R, et al. Higher immunoglobulin E antibody levels to recombinant Fel d 1 in cat-allergic children with asthma compared with rhinoconjunctivitis. Clin Exp Allergy 2008; 38: 1275-1281. https://doi.org/10.1111/j.1365-2222.2008.03003.x
- Konradsen JR, Nordlund B, Onell A, et al. Severe childhood asthma and allergy to furry animals: refined assessment using molecular-based allergy diagnostics. Pediatr Allergy Immunol 2014; 25: 187-192. https://doi.org/10.1111/pai.12198
- Nwaru BI, Suzuki S, Ekerljung L, et al. Furry animal allergen component sensitization and clinical outcomes in adult asthma and rhinitis. J Allergy Clin Immunol Pract 2019; 7: 1230-1238.e4. https://doi.org/10.1016/j.jaip.2018.12.018
- Bjerg A, Winberg A, Berthold M, et al. A population-based study of animal component sensitization, asthma, and rhinitis in schoolchildren. Pediatr Allergy Immunol 2015; 26: 557-563. https://doi.org/10.1111/pai.12422
- Nordlund B, Konradsen JR, Kull I, et al. IgE antibodies to animal-derived lipocalin, kallikrein and secretoglobin are markers of bronchial inflammation in severe childhood asthma. Allergy 2012; 67: 661-669. https://doi.org/10.1111/j.1398-9995.2012.02797.x
- Schoos AM, Nwaru BI, Borres MP. Component-resolved diagnostics in pet allergy: current perspectives and future directions. J Allergy Clin Immunol 2021; 147: 1164-1173. https://doi.org/10.1016/j.jaci.2020.12.640
- Satyaraj E, Wedner HJ, Bousquet J. Keep the cat, change the care pathway: a transformational approach to managing Fel d 1, the major cat allergen. Allergy 2019; 74(Suppl 107): 5-17. https://doi.org/10.1111/all.14013
- Hemmer W. How molecular diagnostics help us to correctly identify pet allergies. Allergo Journal International 2023; 32: 123-129. https://doi.org/10.1007/s40629-023-00255-8
- Curin M, Reininger R, Swoboda I, et al. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol 2011; 154: 258-263. https://doi.org/10.1159/000321113
- Borres MP, Ebisawa M, Eigenmann PA. Use of allergen components begins a new era in pediatric allergology. Pediatr Allergy Immunol 2011; 22: 454-461. https://doi.org/10.1111/j.1399-3038.2011.01197.x
- Huang HJ, Campana R, Akinfenwa O, et al. Microarray-based allergy diagnosis: quo vadis? Front Immunol 2021; 11: 594978. https://doi.org/10.3389/fimmu.2020.594978
- Van Hage M, Hamsten C, Valenta R. ImmunoCAP assays: pros and cons in allergology. J Allergy Clin Immunol 2017; 140: 974-977. https://doi.org/10.1016/j.jaci.2017.05.008
- Bonnet B, Messaoudi K, Jacomet F, et al. An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen. Allergy Asthma Clin Immunol 2018; 14: 14. https://doi.org/10.1186/s13223-018-0239-8
- Grönlund H, Saarne T, Gafvelin G, van Hage M. The major cat allergen, Fel d 1, in diagnosis and therapy. Int Arch Allergy Immunol 2010; 151: 265-274. https://doi.org/10.1159/000250435
- Sekerel BE, Aliyeva G. Advancing diagnostic precision: unveiling sensitization relationships between cat, dog, and horse allergen molecules. Pediatr Allergy Immunol 2024; 35: e14177. https://doi.org/10.1111/pai.14177
- Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol 2009; 123: 362-368. https://doi.org/10.1016/j.jaci.2008.11.021
- Schoos AM, Chawes BL, Bloch J, et al. Children monosensitized to can f 5 show different reactions to male and female dog allergen extract provocation: a randomized controlled trial. J Allergy Clin Immunol Pract 2020; 8: 1592-1597.e2. https://doi.org/10.1016/j.jaip.2019.12.012
- McDonald RE, Fleming RI, Beeley JG, et al. Latherin: a surfactant protein of horse sweat and saliva. PLoS One 2009; 4: e5726. https://doi.org/10.1371/journal.pone.0005726
- Davenport J, Smith D. Equine hypersensitivity: the dark horse of allergy. Clin Rev Allergy Immunol 2020; 59: 352-358. https://doi.org/10.1007/s12016-020-08807-4
- Hellu T, Gomez R, Weiss S, Smith D, Steigelman D. Do commercial dog extracts cross-react with Felis domesticus allergen 1. Allergy Asthma Proc 2024; 45: 447-452. https://doi.org/10.2500/aap.2024.45.240072
- Hilger C, Kuehn A, Hentges F. Animal lipocalin allergens. Curr Allergy Asthma Rep 2012; 12: 438-447. https://doi.org/10.1007/s11882-012-0283-2
- Chruszcz M, Mikolajczak K, Mank N, et al. Serum albumins-unusual allergens. Biochim Biophys Acta 2013; 1830: 5375-5381. https://doi.org/10.1016/j.bbagen.2013.06.016
- Konradsen JR, Fujisawa T, van Hage M, et al. Allergy to furry animals: new insights, diagnostic approaches, and challenges. J Allergy Clin Immunol 2015; 135: 616-625. https://doi.org/10.1016/j.jaci.2014.08.026
- Asarnoj A, Hamsten C, Wadén K, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a BAMSE/MeDALL study. J Allergy Clin Immunol 2016; 137: 813-821.e7. https://doi.org/10.1016/j.jaci.2015.09.052
- Eder K, Becker S, San Nicoló M, Berghaus A, Gröger M. Usefulness of component resolved analysis of cat allergy in routine clinical practice. Allergy Asthma Clin Immunol 2016; 12: 58. https://doi.org/10.1186/s13223-016-0163-8
- Käck U, Asarnoj A, Grönlund H, et al. Molecular allergy diagnostics refine characterization of children sensitized to dog dander. J Allergy Clin Immunol 2018; 142: 1113-1120.e9. https://doi.org/10.1016/j.jaci.2018.05.012
- Hatzler L, Panetta V, Lau S, et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J Allergy Clin Immunol 2012; 130: 894-901.e5. https://doi.org/10.1016/j.jaci.2012.05.053
- Hemmer W, Sestak-Greinecker G, Braunsteiner T, Wantke F, Wöhrl S. Molecular sensitization patterns in animal allergy: relationship with clinical relevance and pet ownership. Allergy 2021; 76: 3687-3696. https://doi.org/10.1111/all.14885
- Van Hage M, Käck U, Asarnoj A, Konradsen JR. An update on the prevalence and diagnosis of cat and dog allergy - emphasizing the role of molecular allergy diagnostics. Mol Immunol 2023; 157: 1-7. https://doi.org/10.1016/j.molimm.2023.03.003
- Kozlov EM, Dubovets AA, Ryabova KA, et al. Modern concept of molecular diagnostics of allergy to dogs. Bull Exp Biol Med 2023; 175: 715-719. https://doi.org/10.1007/s10517-023-05932-w
- Rosada T, Lis K, Bartuzi Z, Grześk-Kaczyńska M, Ukleja-Sokołowska N. Diagnostics of allergy to furry animals-possibilities in 2024. J Clin Med 2024; 13: 3239. https://doi.org/10.3390/jcm13113239
- Riabova K, Karsonova AV, van Hage M, et al. Molecular allergen-specific IgE recognition profiles and cumulative specific IgE levels associated with phenotypes of cat allergy. Int J Mol Sci 2022; 23: 6984. https://doi.org/10.3390/ijms23136984
- MacroArray Diagnostics (MADx). Allergy xplorer (alex²) instruction for use. 2024. Available at: https://a.storyblok.com/f/164899/x/ffbebbc529/02-ifu-01-en-12-ifu-alex.pdf
- Dávila I, Domínguez-Ortega J, Navarro-Pulido A, et al. Consensus document on dog and cat allergy. Allergy 2018; 73: 1206-1222. https://doi.org/10.1111/all.13391
- Dhami S, Agarwal A. Does evidence support the use of cat allergen immunotherapy? Curr Opin Allergy Clin Immunol 2018; 18: 350-355. https://doi.org/10.1097/ACI.0000000000000457
- Smith DM, Coop CA. Dog allergen immunotherapy: past, present, and future. Ann Allergy Asthma Immunol 2016; 116: 188-193. https://doi.org/10.1016/j.anai.2015.12.006
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















