Graphical Abstract
Abstract
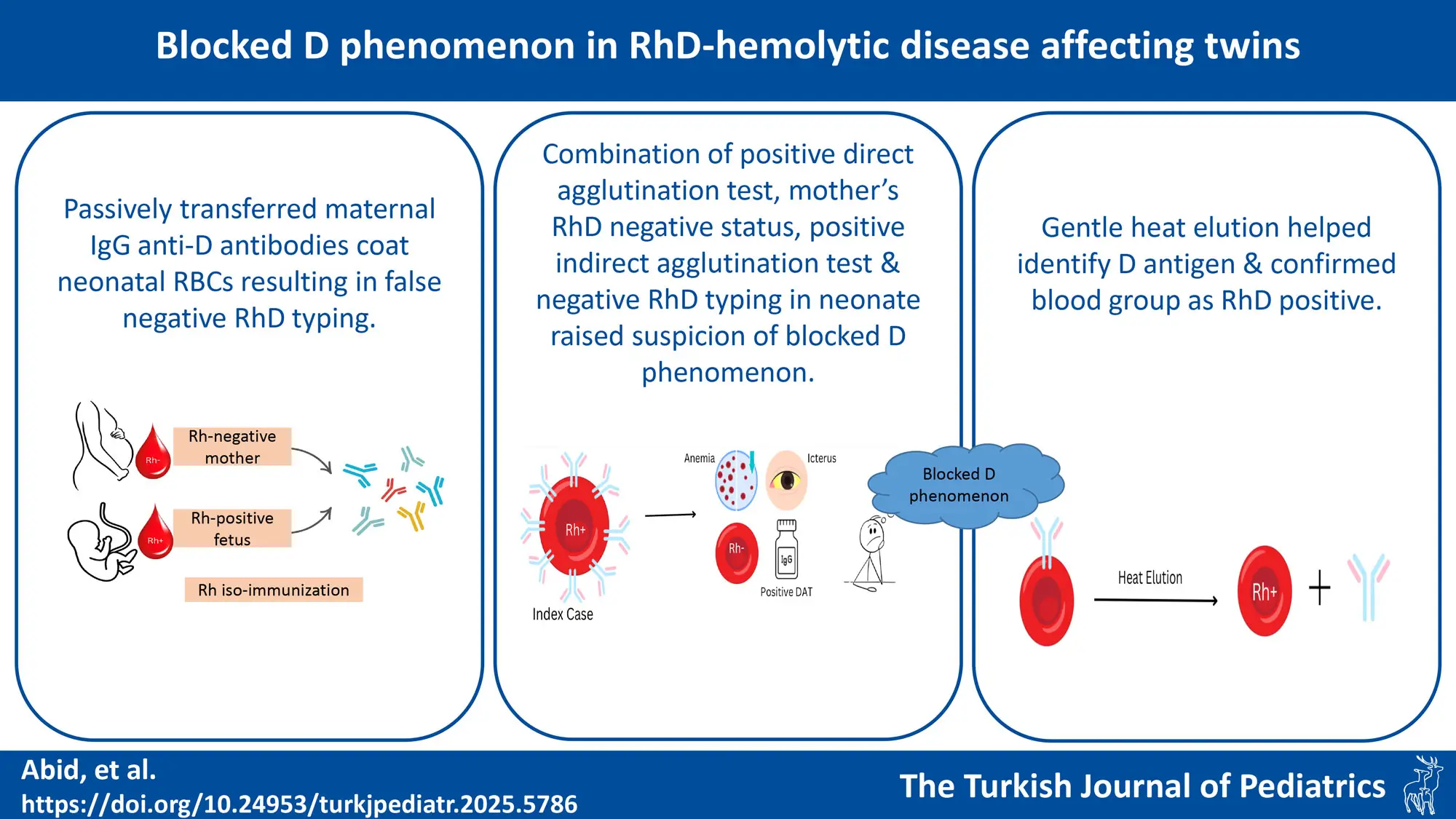
Background. The Rh blood group system is the most common cause of hemolytic disease of the fetus and newborn (HDFN). Rh antigens are fully expressed at birth unlike ABO antigens which are weakly expressed. Sensitization to the D antigen can occur with exposure to < 0.1 mL of fetal blood. In rare cases of HDFN, these passively transferred IgG anti-D antibodies coat the D antigens on the newborn’s red blood cells and interfere with the agglutination of D-positive red cells when tested with IgM anti-D typing reagents, resulting in false-negative Rh(D) typing. This “blocked D phenomenon,” can pose a diagnostic challenge.
Case Presentation. This case report describes twins with HDFN born to a Rh(D) negative mother. Both cord blood and neonatal blood were incorrectly typed as Rh(D) negative using routine typing reagents, creating a diagnostic dilemma. The combination of a positive direct antiglobulin test (DAT), the mother’s RhD-negative status, a positive indirect antiglobulin test (IAT), and discordant or unexpected RhD typing in the neonate raised suspicion of blocked D phenomenon. Paired samples from the parents and neonates were analysed. Following gentle heat elution at 45°C for 10 minutes, the neonatal red cells were re-typed as RhD positive using the conventional tube technique with monoclonal IgM anti-D. At the 6-month follow-up, both infants were phenotyped as O RhD positive.
Conclusions. The possibility of the blocking phenomenon should be considered while interpreting blood group results from fetal or neonatal samples in an alloimmunized pregnancy with potent antibodies. All pregnant women, regardless of their RhD type, should be tested for clinically significant unexpected serum antibodies during pregnancy. Elution methods help in identifying correct D antigen when Rh(D) typing gives uncertain results. Antiglobulin testing with anti-IgG should be performed to detect antibodies causing hemolytic disease of the fetus and newborn (HDFN).
Keywords: Alloantibody, Anti-D antibody, blocking antibody, hemolysis, hyperbilirubinemia, Rh-typing
Introduction
While the incidence of Rhesus hemolytic disease of fetus and newborn (Rh HDFN) has declined significantly with the advent of immune prophylaxis, cases involving other alloantibodies, such as anti-K, have become increasingly common, but still Rh blood group system remains the leading cause of HDFN.1 It is the most complex blood group system in humans, with individuals classified as Rh-positive or Rh-negative based on the presence or absence of the major D antigen on their red blood cells. Although more than 55 Rh antigens have been identified, the most clinically significant ones are D, C, c, E, and e antigens.2
It is well established that no “d antigen” exists, and RhD negativity is characterized by the absence of the D antigen. Production of the D antigen begins as early as 5 weeks of gestation and is completed before birth.3 Pregnant mothers lacking the D antigen can develop anti-D antibodies upon exposure to even a small amount of RhD-positive allogeneic red cells (as little as 0.1 mL).4 HDFN occurs when maternal IgG antibodies cross the placenta and destroy fetal or neonatal red blood cells.5 In rare cases, if a neonate’s red blood cells are heavily coated with passively transferred maternal IgG anti-D antibodies, the coating may interfere with agglutination of D-positive red cells when tested with commercial IgM anti-D typing reagents. This can result in a false-negative Rh(D) typing. This phenomenon, known as the “blocked D phenomenon,” occurs due to the saturation of D antigen sites by the antibodies, preventing detection by routine serological testing.6,7
Here, we present twins with RhD hemolytic disease of the newborn, exhibiting the rare blocked D phenomenon, which resulted in false-negative RhD typing in the neonates.
Case presentation
A 35-year-old second gravida mother delivered dichorionic diamniotic (DCDA) twins at 38 weeks gestation via caesarean section at a local hospital. Her blood group was B Rh negative, while her husband’s blood group was O Rh positive. She had received anti-D immunoglobulin prophylaxis following the birth of her first child 8 years ago. There was no history of blood transfusions during the past 8 years, and her first child did not experience jaundice after delivery. The antenatal period was uneventful, but antibody screening was not conducted at the primary healthcare unit. Both the babies were vigorous at birth and weighed 2680 and 2690 grams respectively. Cord blood group of both twins conducted at the primary unit were reported as O RhD negative.
The twin neonates were referred to our hospital at 22 hours of life due to hyperbilirubinemia. On examination, both babies were icteric up to their palms and soles and were noted to be pale. Vital signs were normal. Laboratory findings for Twin A were consistent with HDFN, including indirect hyperbilirubinemia (total bilirubin: 19.8 mg/dL, direct bilirubin: 1.1 mg/dL, indirect bilirubin: 18.7 mg/dL), anemia (hemoglobin: 11 g/dL) with corrected reticulocyte count of 9% (Table I).8,9 Intensive phototherapy was initiated, and preparations were made for a double-volume exchange transfusion (DVET).
|
Values reported as mean ± standard deviation. The reference values are from: Christensen RD: Expected hematologic values for term and preterm neonates. In Christensen RD, editor: Hematologic Problems of the Neonate, Philadelphia, 2000, Saunders, p. 120.8, and Wu Alan HB. Tietz Guide to Laboratory Tests. 4th ed. Philadelphia: WB Saunders; 2006.9 * Values six hours post double volume exchange transfusion, **Values before packed RBC transfusion, #Values before intravenous immunoglobulin HOL: hours of life |
|||||||||||
| Table I. Laboratory results of Twin A and B in the first 3 days of admission. | |||||||||||
| Parameter | Reference interval |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||
| Hemoglobin (g/dL) | 19.3 + 2.2 |
|
|
|
|
|
|
|
|
|
|
| Hematocrit (%) | 61 + 7 |
|
|
|
|
|
|
|
|
|
|
| Total bilirubin (mg/dL) | < 8 |
|
|
|
|
|
|
|
|
|
|
| Direct bilirubin (mg/dL) | < 0.6 |
|
|
|
|
|
|
|
|
|
|
| Indirect bilirubin (mg/dL) | < 7.4 |
|
|
|
|
|
|
|
|
|
|
| Direct Coombs test | Negative |
|
|
|
|
|
|
|
|
|
|
| Corrected reticulocyte count (%) | 3.2 + 1.4 |
|
|
|
|
|
|
|
|
|
|
Similarly, Twin B also exhibited manifestations of HDFN, presenting with anemia (hemoglobin: 11.3 g/dL) and indirect hyperbilirubinemia (total bilirubin: 19.9 mg/dL, direct bilirubin: 1.2 mg/dL, indirect bilirubin: 18.7 mg/dL) with a corrected reticulocyte count of 11% (Table I).
The indirect antiglobulin test (IAT) performed on maternal blood was positive, with a titer of 1:512 (Anti Human Globulin – Tulip diagnostics). DVET was performed for both twins using O-negative whole blood, following American Academy of Pediatrics (AAP) guidelines. After exchange transfusion, both babies were administered intravenous immunoglobulin (IVIG) due to rising bilirubin level and packed red blood cell transfusions for anemia (Hemoglobin level was 9.1 and 9.3 g/dL for twin A and B respectively).
The peripheral smear of both twins showed polychromasia, anisocytosis, and nucleated red blood cells (RBCs). Both twins were initially typed as O Rh(D) negative using the conventional tube technique with monoclonal IgM anti-D (Biolab Pvt Ltd). The direct antiglobulin test (DAT), conducted using the gel technique (Tulip Matrix Gel system CC 2400), showed a strong positive result (4+).
The combination of a positive DAT, the mother’s RhD-negative status, a positive IAT, and discordant or unexpected RhD typing in the neonate raised suspicion of immune-mediated HDFN, with a potential blocked D phenomenon. Paired samples from the parents and twin babies were sent to the immunohematology department for analysis. Neonatal red cells underwent gentle heat elution at 45°C for 10 minutes. Following elution, the red cells were re-typed as RhD positive using the conventional tube technique with monoclonal IgM anti-D. (Biolab Pvt Ltd). At the 6-month follow-up, blood group of both infants were phenotyped as O RhD positive. Written parental consent was obtained for the publication of the case report.
Discussion
HDFN is characterized by an increased rate of RBC destruction. Diagnostic clues indicating hemolysis include an elevated reticulocyte count, unconjugated hyperbilirubinemia, and characteristic red cell abnormalities on the peripheral smear. Hemolysis in newborns can be categorized into immune-mediated and non-immune-mediated causes, based on its etiology. Immune-mediated hemolysis, such as HDFN, results from maternal antibodies targeting fetal RBC antigens. In contrast, non-immune-mediated causes include conditions like alpha-thalassemia major, hereditary spherocytosis, and glucose-6-phosphate dehydrogenase (G6PD) deficiency.1,5,10
Using the keywords “Alloantibody, anemia, Anti-D antibody, anti-globulin test, blocking antibody, Coombs test, exchange transfusion, hemolysis, hemolytic anemia” we performed a literature search in PubMed (from 1966 until 2024), EMBASE (from 1966 until 2024), and Google Scholar (from 1960 until 2024) and identified 17 case reports of blocked phenomenon involving D, K, and Fya antigens, whose characteristics are shown in Table II. We have identified 12 cases involving a blocking phenomenon caused by D antibodies, 4 cases related to anti-K and anti-C antibodies, and 1 case involving an anti-Fya antibody.
|
*Anti-K 3+, **Both twins had similar results and clinical course, #Rh typing was inconsistent CDP: chloroquine diphosphate, DAT: direct antiglobulin test, ET: exchange transfusion, IAT: indirect antiglobulin test, IVIG: intravenous immunoglobulin, NA: details not available, PT: phototherapy |
|||||||||
| Table II. Laboratory tests and clinical characteristics of Blocked phenomenon cases published in the literature. | |||||||||
| Study | Blood group and Rh typing of mother | Obstetric history | Initial blood group and Rh typing of neonate | Maternal blood test for antibodies | Other antibodies in maternal blood | Infant’s blood test for antibodies | Elution method | Blood group, Rh typing of neonate after Elution | Management |
| Hannon J et al., 200711 | NA | NA | NA | NA | Anti-K | NA | NA | NA | NA |
| Sulochana PV et al., 2008 6 | B RhD negative | G2P2 | B RhD negative |
IAT 4+ Anti D 1:1024 |
Anti-C |
DAT 3+ Anti -D 1:512 |
Heat elution | B RhD positive | 3 ET |
| Moiz B et al., 200812 | O RhD negative | G2P1 | A RhD negative | IAT strong positive | - |
DAT strong + |
CDP | A RhD positive | Packed cell transfusions for anemia |
| Lee E et al., 200913 |
A Rh D+ R(1)R(1) K negative |
G3P3 |
A, R(1)r K negative |
DAT 5+ |
Anti -K 1:256 |
DAT 5+ | CDP |
A, R(1)r K positive |
Mild jaundice |
| Verma A et al., 201314 | AB RhD negative | G10P8 | O RhD negative |
IAT 4+ Anti -D 1:256 |
- | DAT 4+ | CDP | A RhD positive | 3 Intrauterine transfusions |
| Lee E et al., 201515 | A, R1R1 K- Fy(a-) | NA | A, R1R1 K- | NA |
Anti-Fya titre 1:256 |
DAT 3+ | CDP, Flow cytometry, anti-Fya MIMI-19 | A, R1R1 K- Fy(a+b+) | PT |
| Jain A et al., 201516 | AB RhD negative | P2L2 | A RhD negative | Anti -D 1:1024 | Anti-C, anti-S | DAT 4+ | Acid elution, Heat elution | A RhD positive | 3 ET |
| Wang H et al., 201717 | O RhD negative | G2P1 | O RhD uncertain |
IAT 4+ Anti -D 1:2048 |
- | DAT 4+ | Heat elution | O RhD positive |
ET, top up transfusion |
| Manfroi S et al., 201718 | O Rh D positive, R1R1, K-, S+s-, Lu (a-b+) | G2P1L1 | O RhD positive, R1R1, K-, S+s-, Lu (a-b+) | - | Anti-K: 1:1024, anti-s (titre 1), anti-Lu (titre 1) |
DAT 4+ Anti- K 1:256 |
Acid elution | O RhD + K+ |
3 Intrauterine transfusions, PT, 2 top up transfusions |
| Das S et al., 201919 | A RhD negative | G2P1 | O RhD negative | IAT 4+ | Anti-Le | DAT 4+ | Heat elution | O RhD variant type III | IVIG, PT, top up transfusion |
| Subramaniyan R. et al., 201920 | B RhD negative | G3P2L1A1 | B RhD uncertain# |
IAT 4+ Anti -D 1:1024 |
- | DAT 4+ | Heat elution | B RhD positive | IVIG, PT, top up transfusion |
| Mani A et al., 201921 | A RhD negative | G3P1L1A1 | O RhD negative | Anti -D 1:512 |
Anti-C 1:32 |
DAT + | Heat elution | O RhD positive | 4 Intrauterine transfusions |
| B RhD negative | G2P1L1A0 | B RhD negative |
IAT 1:32 |
Anti-C, Anti-E | DAT+ | Heat elution | B RhD positive | Intensive PT | |
| Naik A et al., 202022 | A RhD negative | G2P1 | O RhD negative |
IAT 4+ Anti -D 1:1024 |
Anti-C 1:128 | DAT 4+ | Heat elution, acid elution, CDP | O RhD positive | ET |
| Moosavi M et al., 202023 | K, Jka negative | G4P3 |
O RhD negative K, Jka negative |
- |
Anti-K 1:1024 Anti-Jka 1:4 |
DAT 2+ DAT C3 3+ |
Modified gentle heat elution |
O RhD negative K positive |
ET, IVIG, intensive PT |
| Novoselac J et al., 202024 |
A Rh D positive, K negative |
G5P3A2 |
AB RhD positive, K negative |
Anti-K 3+ |
Anti-K 1:32 |
DAT 3+ | Acid elution |
AB RhD positive, K positive |
PT, 3 top up transfusions |
| Sil S et al., 202225 | A RhD negative | G2P1L1 | AB RhD negative |
Anti-D 1: 128 |
- | DAT 4+ | Acid elution | AB RhD positive | Intensive PT, IVIG |
| Thakkar GH et al., 202426 | B RhD negative | G4P3L2A1 | O RhD negative | Anti -D 1:512 | - | DAT 4+ | Heat elution | O RhD positive |
PT, top up transfusion |
| Present study**, 2025 | B RhD negative | G2P2L1 | O RhD negative |
IAT 4+ Anti -D 1:512 |
- | DAT 4+ |
Saline wash, Heat elution |
O RhD positive | ET, IVIG, intensive PT, top up transfusion |
In the present case, non-immune causes of hemolysis were excluded, as the twins were born to an RhD-negative mother with confirmed RhD incompatibility between the parents, and both babies had a positive direct antiglobulin test (DAT). Initial typing of both twins indicated O Rh(D) negative using the conventional tube technique with monoclonal IgM anti-D (Biolab Pvt Ltd). Given the clinical context and laboratory findings, blocked D phenomenon was suspected. To confirm this, neonatal red cells were subjected to gentle heat elution and post elution blood group was confirmed as O RhD positive. The blocked D phenomenon, characterized by the saturation of RhD antigens with maternal IgG anti-D antibodies, was first demonstrated in vitro by Wiener in 1944.7 The false-negative D typing, or blocked D phenomenon, has been attributed to the prozone effect. This occurs when an excess of antibodies saturates the D antigen sites with anti-D antibodies, preventing their detection. Importantly, the anti-D antibodies do not need to be of a high titer to cause this phenomenon.
Hannon et al.11 first described phenomenon of blocking antibodies due to anti-K in 2007. Sulochana et al.6 described a case of blocked D due to a maternal IgM anti-D titer of 32, with an IgG titer of 1,024. In the same year, Moiz et al.12 described a similar case of blocked D phenomenon, using CDP to elute antibodies.
Similarly, Verma et al.14 reported a case of blocked D in RhD hemolytic disease of the fetus, where at 20 weeks gestation, the maternal anti-D titer was 256, as determined by the conventional tube technique. Wang et al.17 and Subramaniyan et al.20 independently reported cases of blocked D in RhD hemolytic disease of the fetus, where the maternal anti-D titer was 1:1,024. Thakkar et al.26 reported a case of blocked D in RhD hemolytic disease of the fetus with a maternal anti-D titer of 1:512, closely resembling our case. These cases emphasize that high titers of anti-D can result in the blocking phenomenon, potentially leading to misinterpretation of RhD typing.
The blocking phenomenon is not exclusive to anti-D antibodies. Similar false-negative blood typing results have been reported with other antigens. For example, two cases of false-negative K1 typing of fetal cells were attributed to the blocking effect of maternal IgG anti-K antibodies.
Lee et al.13 reported a case of blocking of fetal K antigen, and demonstrated that antenatal anti-K1 samples with a titer of 256 or higher can inhibit K1 antigens. Additionally, Lee et al.15 showed evidence of blocking of Fy antigen sites in a simulated experiment, where high-titer human-murine hybridoma anti-Fy (HIMA-19) antibodies interfered with the detection of Fy antigens. Manfroi et al.18 reported K-antigen blocking phenomenon in a case of HDFN wherein the maternal anti-K titre was 1:1,024. Moosavi et al.23 reported a case of HDFN due to anti-K antibodies (titre 1: 1,024) which was diagnosed using a modified gentle heat elution. These cases underscore that the blocking phenomenon can occur with various blood group antigens, posing challenges to accurate typing and diagnosis.
Novoselac et al.24 reported a case involving anti-K antibodies with a titer of 1:32 that effectively masked K antigens on neonatal red blood cells. The presence of additional alloantibodies, including anti-C, anti-S, and anti-E, in maternal serum alongside anti-D was also documented.16,21,22 Unlike anti-D antibodies, even a low titer of anti-K can block antigens on neonatal red blood cells. Moreover, the antibody titer does not necessarily correlate with the severity of HDFN.24
Das et al.19 reported a variant D phenotype that mimicked blocked D phenomenon. D variants can be classified into two types: weak D and partial D. Partial D antigen variant lacks one or more of the D epitopes whereas weak D antigen variant has all the D epitopes but are expressed weakly. Individuals with partial D antigens may produce anti-D antibodies because they lack certain D epitopes, while individuals with weak D antigens typically do not produce anti-D antibodies, as the full set of D epitopes is still present, albeit weakly expressed. This distinction is important in the context of RhD typing and immunization risk, as weak D antigens may result in misinterpretation of RhD status, while partial D antigens may lead to alloimmunization.19
Upon repeating the Rh typing at six months of life, blood groups of both infants were confirmed to be RhD positive, which was consistent with findings from other studies. However, this result differed from the report by Das et al.19, who identified the case as a weak D variant. The weak D variant typically shows all the D epitopes but with weak expression, which can lead to potential misinterpretation of RhD status in certain settings.
Heat elution, chloroquine diphosphate (CDP), and glycine-EDTA are commonly used methods for eluting antibodies from red blood cells. Heat elution involves exposing red blood cells to a temperature of 56°C to release antibodies. Sil et al.25 used acid elution method to elute antibodies to detect blocked D phenomenon.
CDP, widely used in hematology laboratories, is effective for eluting IgG antibodies while preserving the integrity of the red cell membrane, making it a preferred alternative to heat elution.27 However, Katharia et al.28 found that the glycine-EDTA method is more effective than CDP in reducing the strength of the direct antiglobulin test (DAT) reaction and offers greater accuracy.
If unexpected antibodies are detected during pregnancy, their blood group specificity should be identified. A limited-reagent RBC panel can help exclude clinically significant antibodies other than D. Tests for fetal anemia include amniocentesis, cordocentesis, ultrasound, and Doppler assessment of cerebral artery peak velocity.29 There is limited data on critical titers for non-Rh antibodies in pregnancy. A titer of 64 has been recommended for anti-Fya in HDFN. However, in pregnancies affected by anti-K, antibody titers and amniotic fluid analysis do not reliably predict fetal anemia severity. If there is no fetomaternal ABO incompatibility, maternal serum or infant eluate should be tested against paternal RBCs. Rh-HDFN should not be attributed solely to anti-D, as other alloantibodies may also contribute to hemolysis.30
Conclusions
The possibility of the blocking phenomenon should be considered while interpreting blood group results from fetal or neonatal samples in an alloimmunized pregnancy with potent antibodies. A false-negative RhD grouping can occur if maternal IgG antibodies saturate all available antigen sites on fetal red blood cells, preventing the anti-D reagent from binding. In such cases, a thorough clinical history, details of any intrauterine transfusions and the results of previous immunohematological investigations during the perinatal period, are crucial for accurate diagnosis. All pregnant women, regardless of their RhD type, should be tested for clinically significant unexpected serum antibodies during each pregnancy, ideally during their first visit to the obstetrician. Anti-globulin testing with anti-IgG should be performed to detect antibodies causing hemolytic disease of the fetus and newborn.
Acknowledgements
We thank Department of Transfusion Medicine, NIMS & Department of Pathology, Ankura hospital for Women and Children, Hyderabad for performing the tests.
Ethical approval
This case report does not require approval of Ethics committee. Written informed consent of parents was obtained. The identity of patients was not disclosed in the manuscript.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Myle AK, Al-Khattabi GH. Hemolytic disease of the newborn: a review of current trends and prospects. Pediatric Health Med Ther 2021; 12: 491-498. https://doi.org/10.2147/PHMT.S327032
- Zhao Y, Yao N, Lv Y, Cui D, Xie J. Analysis of Rhesus (Rh) antigen distributions in donors and multi-transfused patients for phenotype-matched transfusion. Indian J Hematol Blood Transfus 2024; 40: 130-138. https://doi.org/10.1007/s12288-023-01676-9
- McBain RD, Crowther CA, Middleton P. Anti-D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev 2015; 2015: CD000020. https://doi.org/10.1002/14651858.CD000020.pub3
- Bowman JM. The prevention of Rh immunization. Transfus Med Rev 1988; 2: 129-150. https://doi.org/10.1016/s0887-7963(88)70039-5
- Urbaniak SJ, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev 2000; 14: 44-61. https://doi.org/10.1054/blre.1999.0123
- Sulochana PV, Rajesh A, Mathai J, Sathyabhama S. Blocked D phenomenon, a rare condition with Rh D haemolytic disease of newborn - a case report. Int J Lab Hematol 2008; 30: 244-247. https://doi.org/10.1111/j.1751-553X.2007.00943.x
- Wiener AS. A new test (blocking test) for Rh sensitization. Proc Soc Exp Biol Med 1944; 56: 173-176. https://doi.org/10.3181/00379727-56-14640
- Christensen RD. Expected hematologic values for term and preterm neonates. In: Christensen RD, editor. Hematologic problems of the neonate. Philadelphia: Saunders; 2000: 120.
- Wu Alan HB. Tietz guide to laboratory tests. 4th ed. Philadelphia: WB Saunders; 2006.
- Christopher ST, Lambert MP. Hemolytic disease of the newborn. In: Kliegman RM, Geme JW, editors. Nelson Textbook of Pediatrics. 22nd ed. 2024: 1123-1125.
- Hannon J, Clarke G, Caruk B, Button E. Blocking phenomenon due to anti-Kell in post-natal investigation. Transfus Med 2007; 17: ABS26.
- Moiz B, Salman M, Kamran N, Shamsuddin N. Transfusion medicine illustrated: blocked D phenomenon. Transfusion 2008; 48: 1545-1546. https://doi.org/10.1111/j.1537-2995.2008.01773.x
- Lee E, Redman M, Owen I. Blocking of fetal K antigens on cord red blood cells by maternal anti-K. Transfus Med 2009; 19: 139-140. https://doi.org/10.1111/j.1365-3148.2009.00917.x
- Verma A, Sachan D, Bajpayee A, Elhence P, Dubey A, Pradhan M. RhD blocking phenomenon implicated in an immunohaematological diagnostic dilemma in a case of RhD-haemolytic disease of the foetus. Blood Transfus 2013; 11: 140-142. https://doi.org/10.2450/2012.0005-12
- Lee E, Cantwell C, Muyibi KO, Modasia R, Rowley M, New H. Blocking phenomenon occurs with murine monoclonal antibodies (anti-Fy(a)) in a neonate with a positive direct antiglobulin test due to maternal anti-Fy(a). Blood Transfus 2015; 13: 672-674. https://doi.org/10.2450/2015.0232-14
- Jain A, Kumawat V, Marwaha N. Blocked D phenomenon and relevance of maternal serologic testing. Immunohematology 2015; 31: 116-118.
- Wang H, Chen J, Jiang Y. A case of a newborn with blocked RhD antigen and HDFN. Lab Med 2017; 48: 381-383. https://doi.org/10.1093/labmed/lmx061
- Manfroi S, Velati C. K-antigen blocking in a case of haemolytic disease of the foetus and newborn. Blood Transfus 2017; 15: 585-586. https://doi.org/10.2450/2017.0254-16
- Das S, Shastry S, Baliga PB. Severe haemolytic disease of a newborn with variant D mimicking blocked-D phenomenon. BMJ Case Rep 2019; 12: e231891. https://doi.org/10.1136/bcr-2019-231891
- Subramaniyan R. Blocked D in RhD hemolytic disease of fetus and newborn. Glob J Transfus Med 2019; 4: 114-116. https://doi.org/10.4103/GJTM.GJTM_54_18
- Mani A, Poornima AP, Gupta D. Blocked-D phenomenon in hemolytic disease of fetus and newborn with multiple maternal anti-rhesus antibodies. Hematol Transfus Int J 2019; 7: 35-39. https://doi.org/10.15406/htij.2019.07.00202
- Naik A, Bhattacharya P, Datta SS. Blocking phenomenon occurs in a neonate with a positive direct antiglobulin test due to maternal anti-D, anti-C antibodies: resolved by chloroquine diphosphate treatment. Indian J Hematol Blood Transfus 2020; 36: 403-405. https://doi.org/10.1007/s12288-019-01190-x
- Moosavi M, Ma Y, Baez J, et al. Resolving blocked antigen phenomenon in hemolytic disease of the fetus and newborn due to anti-K. Transfus Med Rev 2020; 34: 124-127. https://doi.org/10.1016/j.tmrv.2020.02.002
- Novoselac J, Raos M, Tomac G, Lukić M, Golubić Ćepulić B. K antigens on neonatal red blood cells blocked by anti-K with titer of 32. Immunohematology 2020; 36: 54-57.
- Sil S, Kaur D, Jain A, et al. D or anti-D!!! Unblocking the dilemma of blocking-D phenomenon using acid elution. Transfus Apher Sci 2022; 61: 103443. https://doi.org/10.1016/j.transci.2022.103443
- Thakkar GH, Shah MC, Bhatnagar NM, Shah SD. Severe haemolytic disease of newborn with possibility of blocked D phenomenon: a case report. BJKines National Journal of Basic & Applied Sciences 2021; 16: 70-72.
- Burin des Roziers N, Squalli S. Removing IgG antibodies from intact red cells: comparison of acid and EDTA, heat, and chloroquine elution methods. Transfusion 1997; 37: 497-501. https://doi.org/10.1046/j.1537-2995.1997.37597293880.x
- Katharia R, Chaudhary RK. Removal of antibodies from red cells: comparison of three elution methods. Asian J Transfus Sci 2013; 7: 29-32. https://doi.org/10.4103/0973-6247.106727
- Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. N Engl J Med 2000; 342: 9-14. https://doi.org/10.1056/NEJM200001063420102
- Judd WJ; Scientific Section Coordinating Committee of the AABB. Practice guidelines for prenatal and perinatal immunohematology, revisited. Transfusion 2001; 41: 1445-1452. https://doi.org/10.1046/j.1537-2995.2001.41111445.x
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















