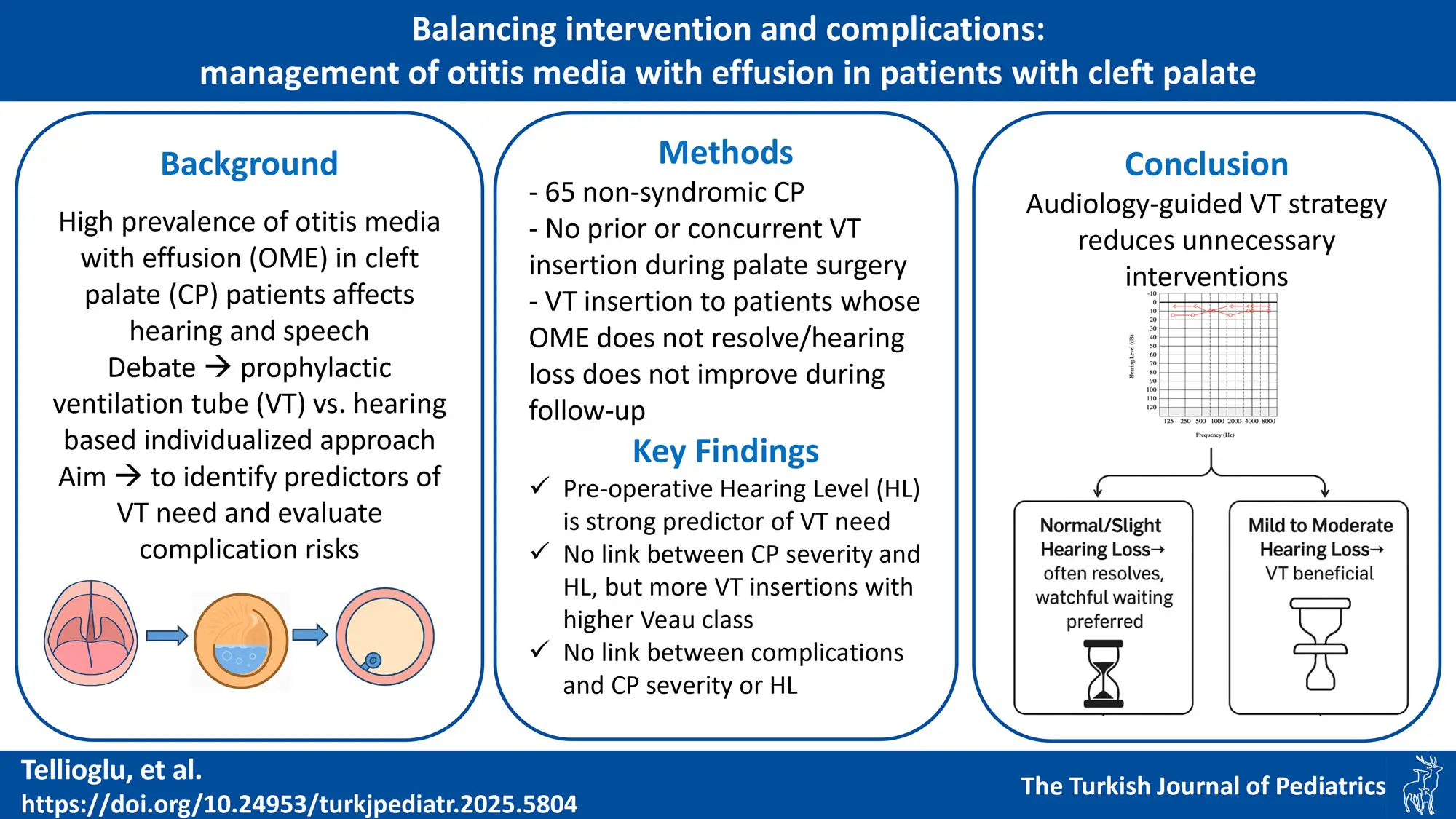
Graphical Abstract
Abstract
Background. Children with cleft palate (CP) are at high risk for otitis media with effusion (OME), which may impair hearing, speech, and development. Although ventilation tube (VT) insertion during palatoplasty is common, its universal use is debated due to uncertain long-term benefits and potential complications. This study aimed to identify preoperative audiological predictors of VT necessity and evaluate VT-related complications.
Methods. A retrospective review was conducted on 65 non-syndromic CP patients who underwent palatal repair without prior or concurrent VT placement. Preoperative audiological evaluations were performed, and patients were followed postoperatively for VT insertion and complications. Preoperative hearing thresholds, cleft severity (Veau classification), and VT related complications were analyzed statistically.
Results. The likelihood of VT insertion rose significantly in parallel with the severity of preoperative hearing loss, ranging from just 5.9% in patients with normal hearing to 75% in those with moderate conductive hearing loss (CHL) (p < 0.001). Pairwise comparisons showed significant differences between normal hearing and both mild (p = 0.0026) and moderate CHL (p = 0.01). CP severity was not associated with preoperative hearing but correlated with higher VT placement (Veau I: 10%, Veau IV: 69.2%; p = 0.035). Complications included otorrhea (45.2%), early extrusion (35.5%), and tympanic membrane perforation (12.9%), with no significant associations to preoperative hearing level and CP severity.
Conclusion. Preoperative hearing level at the time of palate repair is a strong predictor of VT need in CP patients. Mild to moderate CHL significantly increases the risk of persistent OME, supporting early intervention. Normal or slight loss often resolves without treatment, favoring a conservative approach. Higher cleft severity is associated with increased VT placement rates; it does not correlate with preoperative hearing levels or increased VT-related complications. These findings highlight the value of individualized, hearing-based decisions over routine tube placement.
Keywords: cleft palate, otitis media with effusion, ventilation tube, audiology, conductive hearing loss
Introduction
Cleft lip with or without cleft palate (CLP) represents one of the most prevalent congenital anomalies, with an incidence ranging from 1/500 to 1/1000 births.1 Children with cleft palate (CP) are at increased risk of developing otitis media, with rates approaching 100%.2 A decrease in eustachian tube function, particularly an impairment in the opening function, results from malfunction of the tensor veli and levator veli palatini muscles, which are compromised in patients with CP. This causes a disruption in the middle ear airflow, which facilitates the development of otitis media with effusion (OME). OME is well-documented to impede speech and language development, underscoring the critical need for accurate diagnosis and prompt treatment in patients with CP.3-5
The standard approach to addressing OME in patients with CP involves the insertion of ventilation tubes (VT) during CP repair procedures.6 VT insertion is mostly performed to restore hearing immediately in order to prevent or minimize developmental impairment in children with OME.7 However, recent reports suggest that not all patients with OME require VTs during palate surgery, as the procedure itself has demonstrated the capacity to improve middle ear ventilation and Eustachian tube function.8,9 Thus, the meticulous selection of patients who are suitable candidates for VT insertion is paramount for healthcare providers and patients alike, aiming to mitigate potential complications such as recurrent otorrhea, permanent alterations to the tympanic membrane, and iatrogenic cholesteatoma formation.10-13 Furthermore, research indicates that prophylactic VT insertion may not offer significant advantages over vigilant monitoring of middle ear status in patients with CP and OME.14 The current trend leans towards a ‘wait-and-see’ approach regarding middle ear effusion, steering away from prophylactic VT insertion during palate surgery.11,15-17
This study aimed to assess the significance of ontological and audiological findings in patients who had undergone palate surgery, with the objective of identifying potential candidates for VT insertion.
Materials and Methods
This article is a retrospective case review, and all interventions were performed in the Departments of Audiology, Otolaryngology-Head and Neck Surgery, and Plastic and Reconstructive Surgery at Hacettepe University Hospital, a tertiary reference center. The study was approved by the Hacettepe University Ethics Committee for Non-Interventional Clinical Investigations with the number 21/859.
Patients
A retrospective review was conducted on the medical records of patients with CP who underwent surgery at our institution between February 2016 and September 2021. Inclusion criteria required children (<18 years) with CP not associated with a genetic syndrome, to have undergone palate repair, to have completed regular otological and audiological follow-up, and to have completed at least one year of follow-up. Exclusion criteria included patients with isolated cleft lip, those who had a VT placed prior to or during palate surgery, those with sensorineural or mixed hearing loss, those with unilateral hearing loss, and those who did not meet the one-year follow-up requirement. Patients were excluded if they had syndromic CP, as this condition is frequently linked to craniofacial skeletal abnormalities that increase susceptibility to multifactorial middle ear disease and other etiologies of hearing loss. A total of 65 patients with CP who met these criteria were included in the study.
Interventions
All newborns, including those with CP, were screened with the automatic auditory brainstem response (AABR) test using the MB 11 BERAphone (CE certificate 0123, Berlin, Germany) shortly after birth as part of the nationwide screening program. Infants with CP are at increased risk of conductive hearing loss, often due to middle ear effusion. Therefore, comprehensive audiological evaluations at 3 and 6 months of age are recommended, even if the initial newborn screening is passed. For patients with CP who fail the screening test, diagnostic tests such as air and bone conduction ABR and behavioral observation audiometry have been performed at around 3 months of age. Patients continued to receive audiological evaluation every 3 months until palate surgery. The patients included in the study consisted of patients who did not receive a VT before or during palate repair. The last audiological evaluations of the patients before palate repair were noted. The relationship between hearing levels before palate repair and the rate of VT placement during follow-up was analyzed. Early extrusion, need for re-tube placement, VT-related otorrhea, permanent perforation and cholesteatoma development were noted in the follow-up of patients with VT placement.
CP were classified according to the Veau classification.18 According to Veau, defects of the soft palate only are classified as Group I, defects involving the hard palate and soft palate are classified as Group 2, defects involving the soft palate up to the alveolus, usually with involvement of the lip, are classified as Group 3, and complete bilateral clefts are classified as Group 4. Between months 9 and 12, patients underwent palate repair at the Department of Plastic and Reconstructive Surgery. Furlow palatoplasty was preferred in Veau I clefts, given its adequacy for narrow defects; however, in cases with wider clefts, Dorrance palatoplasty was employed due to insufficient tissue mobilization with the Furlow method alone. For Veau II clefts, Dorrance was the standard approach, but in clefts too wide for tension-free closure, a two-flap palatoplasty was used. Conversely, a minority of patients with narrow Veau II clefts underwent primary repair without flap elevation. In more complex Veau III and IV clefts, two-flap palatoplasty was routinely selected, as it allows for greater tissue mobilization and tension-free closure, which simpler techniques could not achieve. These decisions were made on a case-by-case basis following intraoperative assessment of cleft width and tissue characteristics.
Otological examination and pneumatic otoscopy findings were complemented with impedance and audiometric measurements. GSI TympStar Version 1 (CE certificate 0344; Smørum, Denmark) was used to measure middle ear impedance. Type A tympanograms, with their peak pressures within -50 daPa and compliance ranging from 0.2 to 1.4 cc, were considered normal. Pathological curves included tympanograms Ad, As, B, and C.
For children older than 5 years of age, pure tone averages (PTA) at 500, 1000, 2000, and 4000 Hz were calculated, and the level of hearing loss was classified based on Clark’s evaluation19: 0–15 dB as normal hearing, 16–25 dB as slight hearing loss, 26–40 dB as mild hearing loss, 41–55 dB as moderate hearing loss, 56–70 dB as moderately severe hearing loss, 71–90 dB as severe hearing loss, and more than 91 dB as profound hearing loss.
Statistical analysis
Data analysis was performed using SPSS 25 (SPSS Inc., Chicago, IL, USA). The chi-square (χ2) test was utilized to investigate the relationship between categorical variables. Post-hoc analysis with Bonferroni correction was employed to compare every subset further. Subgroup analysis utilized the phi test, and Cramer’s V was used to calculate effect sizes in a 4x2 table. Effect sizes of 0.1, 0.3, and 0.5 were classified as small, medium, and large, respectively. A p-value below 0.05 indicated statistical significance.
Results
Patient demographics
Of the patients included in the study, 60% (n=39) were male and 40% (n=26) were female. The age of the patients at the time of palate surgery ranged from 8 to 35 months, with a mean age of 10.7±4 months. Of the patients, 15.4% (n=10) had Veau type 1, 33.8% (n=22) had Veau type 2, 30.8% (n=20) had Veau type 3 and 20% (n=13) had Veau type 4 CP anomalies. The most preferred surgical technique for palate repair in patients with Veau 1 CP was Furlow palatoplasty (90%, n=9), in patients with Veau 2 CP it was Dorrance palatoplasty (54.5%, n=12), and in patients with Veau 3 CP and Veau 4 CP it was two flap palatoplasty (95%, n=19 and 100%, n=13 respectively). Preoperative hearing levels were normal in 26.1% of patients (n=17), while 15.4% of patients (n=10) had bilateral slight conductive hearing loss (CHL), 46.2% of patients (n=30) had bilateral mild CHL, and 12.3% of patients (n=8) had bilateral moderate CHL. VTs were not inserted during the follow-up period, as the hearing of 52.3% of patients (n=34) remained within normal limits or the hearing of patients with hearing loss returned to normal. However, a VT was placed in 47.7% of patients (n=31) because their hearing did not improve, or their hearing loss progressed. While 30.8% of patients (n=20) had a VT placed only once, 16.9% of patients (n=11) required VT insertion more than once because the VT extruded early, and OME persisted (Table I).
| CHL: conductive hearing loss, CP: cleft palate | |||
| Table I. Patient demographics, n (%). | |||
| Gender | Male | 39 (60%) | |
| Female | 26 (40%) | ||
| CP type | Veau 1 | 10 (15.4%) | |
| Veau 2 | 22 (33.8%) | ||
| Veau 3 | 20 (30.8%) | ||
| Veau 4 | 13 (20%) | ||
| Hearing levels before CP Repair | Bilateral normal hearing | 17 (26.1%) | |
| Bilateral slight CHL | 10 (15.4%) | ||
| Bilateral mild CHL | 30 (46.2%) | ||
| Bilateral moderate CHL | 8 (12.3%) | ||
| Ventilation tube insertion | No | 34 (52.3%) | |
| Yes | Only once | 20 (30.8%) | |
| More than once | 11 (16.9%) | ||
CP type and its effect on preoperative hearing levels and progression of hearing loss
The percentage of patients with normal hearing before CP repair was 50% (n=5), 27.3% (n=6), 15% (n=3) and 23.1% (n=3) in the Veau 1, 2, 3 and 4 groups, respectively. No significant relationship was found between the CP type and the preoperative hearing levels of the patients (χ2=4.83 and p=0.848, Table II). After CP surgery, VT was placed in 10% (n=1), 45.5% (n=10), 55% (n=11) and 69.2% (n=9) of patients in the Veau 1, 2, 3 and 4 groups, respectively, because hearing did not improve or worsened. A significant relationship was found between the severity of the CP and the insertion of VT during the patients’ follow-up (χ2=8.585 and p=0.035, Table II).
| Chi-square test, *p<0,05 accepted as statistically significant, Cramer’s V represents strength of association, HL: hearing loss. | ||||||||||||
| Table II. Hearing status before palatal repair and rates of ventilation tube insertion by cleft palate type. | ||||||||||||
| Cleft palate type |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||
| Veau 1 |
|
|
|
|
|
|
|
|||||
| Veau 2 |
|
|
|
|
|
|
|
|||||
| Veau 3 |
|
|
|
|
|
|
|
|
|
|
|
|
| Veau 4 |
|
|
|
|
|
|
|
|||||
Effect of hearing levels before CP surgery on VT insertion rate
All patients underwent an otological examination, tympanometry and age-appropriate audiometric testing (including bone conduction ABR) prior to palate surgery. While 26.1% of patients (n=17) had normal hearing, 73.9% of patients (n=48) had varying degrees of CHL (Table I). VTs were placed during follow-up in 5.9% (n=1) of patients with normal hearing, 50% (n=5) of those with slight hearing loss, 63.3% (n=19) of those with mild hearing loss and 75% (n=6) of those with moderate hearing loss (Table III). A significant and strong relationship was found between the level of hearing loss in patients before CP surgery and the insertion of VTs during follow-up (χ2=17.26, p=0.0006, Cramer’s V: 0.515, Table III). In other words, as the degree of hearing loss prior to CP surgery increased, the likelihood of OME resolution decreased and the need for VTs increased. In pairwise comparisons, there was a significant difference in the need for tube insertion between normal vs. mild HL (χ2=12.39; p=0.0026, phi=0.513) and normal vs. moderate HL (χ2=9.69, p=0.01, phi=0.622, Table IV).
| Chi-square test, *p<0.05 accepted as statistically significant, Cramer’s V represents strength of association, HL: hearing loss. | |||||
| Table III. Rates of ventilation tube insertion during follow-up of patients by hearing level of patients before palatal surgery. | |||||
| Hearing Level |
|
|
|
|
|
|
|
|
||||
| Bilateral normal |
|
|
|
|
|
| Bilateral slight HL |
|
|
|||
| Bilateral mild HL |
|
|
|||
| Bilateral moderate HL |
|
|
|||
| Chi-square test, the adjusted p cut-off for raw p was accepted as 0.0083 (0.05/6). *p<0.05 accepted as statistically significant for p-values corrected by Bonferroni method, Phi represents strength of association, HL: hearing loss. | ||||
| Table IV. Pairwise comparison of ventilation tube insertion rates by hearing level of patients before palatal surgery. | ||||
| Pairwise Comparisons |
|
|
|
|
| Normal vs. slight HL |
|
|
|
|
| Normal vs. mild HL |
|
|
|
|
| Normal vs. moderate HL |
|
|
|
|
| Slight vs. mild HL |
|
|
|
|
| Slight vs. moderate HL |
|
|
|
|
| Mild vs. moderate HL |
|
|
|
|
VT-related complications
The follow-up period of the 47.7% of patients (n=31) who had a VT inserted ranged from 12 to 118 months. The median follow-up was 42 months. No significant relationship was found between cleft severity and VT complications (Table V). Although early extrusion was more frequently observed in patients with greater preoperative HL, the association was not statistically significant (p = 0.118). Similarly, no significant correlations were found between hearing levels and otorrhea (p = 0.706) or persistent perforation (p = 0.443, Table VI). In 16.9% of patients (n=11), the VT extruded early before the OME resolved and these patients required more than one episode of VT insertion. These patients required an average of 2.82 VT insertions. 45.2% of patients (n=14) had at least one episode of VT otorrhea. 12.9% of patients (n=4) had a permanent perforation of the tympanic membrane after extrusion of the VT (Table V). In all reported cases, the VT had extruded spontaneously rather than being removed surgically. The diagnosis of “permanent perforation” was made at least 12 months after extrusion, during routine postoperative follow-up. All 4 patients who developed a persistent perforation were found to have had at least one episode of VT otorrhea.
| χ2: Chi-square test, *p<0.05 accepted as statistically significant. | |||||||||||
| Table V. Frequency of complications in patients with ventilation tubes by cleft palate type. | |||||||||||
| Complication |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
n (%) |
n (%) |
||
| Early extrusion |
|
|
|
|
|
|
|
|
|
|
|
| Otorrhea |
|
|
|
|
|
|
|
|
|
|
|
| Permanent perforation |
|
|
|
|
|
|
|
|
|
|
|
| Cholesteatoma |
|
|
|
|
|
|
|
|
|
|
|
| χ2: Chi-square test, *p<0.05 accepted as statistically significant, HL: hearing loss. | |||||||||||
| Table VI. Frequency of complications in patients with ventilation tubes by pre-operative hearing level. | |||||||||||
| Complication |
|
|
|
|
|
|
|||||
|
(n) |
(n) |
|
|
|
|
(n) |
(n) |
n (%) |
n (%) |
||
| Early extrusion |
|
|
|
|
|
|
|
|
|
|
|
| Otorrhea |
|
|
|
|
|
|
|
|
|
|
|
| Permanent perforation |
|
|
|
|
|
|
|
|
|
|
|
| Cholesteatoma |
|
|
|
|
|
|
|
|
|
|
|
Discussion
CP type and its effect on hearing status and progression
It is reasonable to expect that more severe CP types (e.g., BCLP, Veau IV) would be associated with poorer initial hearing status and less favorable OME prognosis.6 Previous studies have indicated that a higher proportion of patients with complete clefts require repeat VT placements compared to those with incomplete clefts.7,20,21 Conversely, Shaffer et al. found no association between multiple VT insertions and CP type or Veau classification.22 Similarly, Nomura et al. observed no correlation between the recurrence of OME after palatoplasty and CP type.23
In our study, although a statistically significant increase in VT insertion rates was observed with higher Veau classifications (from 10% in Veau I to 69.2% in Veau IV; p = 0.035), we found no direct association between cleft severity and preoperative hearing levels or complication rates. This partially aligns with the findings of Iemura-Kashiwagi et al., who reported that patients with more extensive clefts—particularly those involving the alveolus—were at greater risk of OME recurrence and thus more likely to require repeated tympanostomy tube insertion.6 However, Schwarz et al. found no statistically significant correlation between cleft width, cleft type, and VT insertion prevalence, suggesting that additional anatomical or functional factors may influence surgical decisions.24 Yoshitomi et al. further supported this by showing that cleft width was significantly associated with the severity and nature of middle ear effusion prior to palatoplasty, but not with the overall incidence or duration of OME.25 Together, these findings emphasize the complexity of predicting VT needs based solely on cleft morphology and support the use of audiological criteria—such as hearing thresholds—as a more reliable indicator, as proposed in our individualized management strategy.
Prophylactic (early) vs. late grommet insertion
Several studies have demonstrated the beneficial effects of early tympanostomy tube placement on hearing, speech, and language development in children with CP, supporting a proactive approach to the management of OME.4,21,26-28 However, other researchers have raised concerns regarding this strategy, citing potential complications such as myringosclerosis, tympanic membrane perforation, and cholesteatoma formation.9,11,29,30
Proponents of early tympanostomy tube placement include Frisina et al. who identified the absence of a VT at the time of palate repair as an independent prognostic risk factor for hearing loss in patients with CP.31 Similarly, Azman et al. reported favorable otological outcomes in younger children who underwent selective VT insertion during palatal closure before the age of one.32 Valtonen et al. demonstrated that early tympanostomy performed at six months of age yields comparable otological and audiological outcomes, as well as mastoid air cell system development, in both cleft and non-cleft patients with OME, without significant long-term otologic complications.33 Klockars et al. suggested that early VT placement, even before palatal closure, may offer better outcomes.34 Inoue et al. evaluated the long-term otological and audiological outcomes in children with and without CP who underwent tympanostomy for OME before the age of 2.35 They concluded that outcomes were similar in both groups, affirming the positive effects of VT on hearing and language development in patients with OME.
Conversely, Robson et al. reported no significant advantage of early VT on developmental outcomes and even observed worse hearing in the treated group, supporting a conservative approach.11 In their retrospective series of 213 CP patients with OME, Gani et al. reported that they placed VT in only 41 patients (19.2%), 22 at the time of palatal surgery and 19 at follow-up, and treated the remaining 22 patients (10.3%) with hearing aids.30 In another study, a conservative approach resulted in a 29% tube insertion rate, with more frequent grommet use observed in patients with severe clefts.21 These findings indicate that prophylactic VT placement is not universally required for all patients with CP. Systematic reviews by Ponduri et al. and Kuo et al. concluded that evidence for early VT benefits remains limited.9,29 Maina et al., in their recent review, emphasized that while VT insertion may be associated with increased complication rates, conservative management can be a safe alternative when hearing is closely monitored.36
It is intuitively plausible that CP repair may improve Eustachian tube function by restoring the integrity of the palatal muscles and soft palate9, Supporting this, D’Andrea et al. observed that early interventions, such as Sommerlad intravelar veloplasty, reduced the need for VT insertions by decreasing persistent OME.8 Additionally, some studies indicate that as Eustachian tube function matures with age, patients with CP require fewer VT insertions later in life.37 However, the majority of patients with CP develop OME at an early age, making early intervention critical to ensuring their hearing, speech, and motor development progress in line with their peers.32,38,39 At this stage, it is imperative to establish clear, evidence-based indications to determine which patients would benefit from VT placement and the optimal timing for intervention.
A recent guideline clearly stated that VT insertion may not be required for all CP patients and should be based on tympanic membrane status and hearing loss assessment prior to palatoplasty.40 In line with this, our data suggest that audiological tests are reliable tools for differentiating risk groups and should be included in the armamentarium of every clinician treating these patients. Patients, especially those with mild and moderate hearing loss, could benefit from VT insertion during CP surgery. Because these patients are less likely to experience resolution of the effusion compared to patients with normal hearing. In patients with hearing loss less than 25 dB, watchful waiting may be a more appropriate approach to avoid complications of unnecessary VT insertion.
Complications of VT in patients with CP
VT complication rates may reach 80%, with otorrhea being the most common and burdensome.41 Studies indicate that otorrhea rates are notably higher in patients with CP compared to those without.29 Ungkanont indicated that the group undergoing routine VT insertion exhibited a greater prevalence of tympanic membrane abnormalities and an increased number of grommets placed.42
Conversely, a large-scale study involving 3,003 patients found no statistically significant differences in complication rates, including otorrhea or the need for ear nose throat (ENT) follow-ups, between CP patients with tube insertion and non-CP patients.43 Similarly, a retrospective study of 285 patients reported a low rate (7.5%) of persistent tympanic membrane perforation following VT insertion, with only 3 cases of cholesteatoma due to tympanic membrane retraction.31 Another study with 116 patients and a 72-month follow-up concluded that VT insertion did not influence cholesteatoma development in patients with CP.44
In line with the literature, we found that the most common complication of VT was otorrhea with rates of 45.2% (n=14). In our study, the median follow-up of patients who had ventilation tubes placed was 42 months, and no patients developed cholesteatoma. However, it was found that 12.9% of patients placed in VT developed a permanent perforation. The reason why this rate is higher than in the literature may be due to the use of Paparella type 2 VTs. In our clinic, because of the longer duration of effusion and the higher need for repeated VTs in patients with CP, medium to long term Paparella type 2 tympanostomy tubes are preferred. It is known that permanent perforation rates increase as the duration of VT increases.23
In our study, no significant relationship was found between the type of cleft palate and VT-related complications. Although the results of the study by Shaffer et al.22 are parallel to this finding, there are articles in the literature arguing that the early extrusion rate increases with increasing cleft palate severity.7,21 Our analysis showed that while complication rates, particularly early extrusion, appeared higher in patients with moderate hearing loss, no statistically significant relationships were established between preoperative hearing levels and VT-related complications. Although several studies have examined the timing of VT placement and its impact on hearing outcomes in patients with cleft palate, there is a lack of research specifically investigating the direct relationship between preoperative hearing levels and VT-related complications. Therefore, further studies are warranted in this area.
Limitations
The study adds valuable information to the ongoing debate about prophylactic versus selective VT insertion during CP repair, emphasizing evidence-based patient selection. The study has several limitations. The heterogeneity in surgical techniques, including Furlow palatoplasty, Dorrance palatoplasty, and two-flap palatoplasty, may have influenced outcomes differently due to varying effects on Eustachian tube function and middle ear ventilation. The limited sample size reduces the statistical power and may obscure significant differences or relationships between subgroups. Additionally, the retrospective design introduces potential bias and limits the ability to establish causality. As a single-center study, the findings may not be generalizable to other populations or healthcare settings. The use of medium-to-long-term Paparella type 2 tympanostomy tubes, which are associated with higher rates of permanent perforation, may have influenced the reported complication rates, limiting the comparability of the results with studies using short-term tubes.
Conclusion
This study underscores the importance of individualized management strategies for OME in patients with CP. Audiological assessments proved critical for stratifying risk groups and identifying candidates for VT insertion. While mild to moderate hearing loss was significantly associated with persistent OME and the need for VTs, patients with normal or slight hearing loss benefited from a conservative approach, minimizing unnecessary interventions and related complications. In addition, the literature presents some inconsistencies; however, it can be reasonably inferred that individuals with more severe cleft deformities are less likely to achieve resolution of OME. Such cases may necessitate repeated VT insertions and, therefore, demand closer and more frequent monitoring.
Tailoring VT insertion to patients’ audiological profiles may optimize outcomes by balancing the benefits of early intervention against potential complications. Further prospective studies are needed to refine these guidelines and evaluate long-term impacts on hearing, speech, and language development.
Acknowledgements
We would like to express our gratitude to all the nursing staff and surgeons of the Department of Otorhinolaryngology Head and Neck Surgery and Department of Plastic and Reconstructive Surgery, Hacettepe University Hospital, School of Medicine for their assistance.
Ethical approval
The study was approved by Hacettepe University Non-Interventional Clinical Investigations Ethics Committee (date: 29.06.2021, number: 21/859).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Kapitanova M, Knebel JF, El Ezzi O, Artaz M, de Buys Roessingh AS, Richard C. Influence of infancy care strategy on hearing in children and adolescents: a longitudinal study of children with unilateral lip and /or cleft palate. Int J Pediatr Otorhinolaryngol 2018; 114: 80-86. https://doi.org/10.1016/j.ijporl.2018.08.031
- Sheahan P, Miller I, Sheahan JN, Earley MJ, Blayney AW. Incidence and outcome of middle ear disease in cleft lip and/or cleft palate. Int J Pediatr Otorhinolaryngol 2003; 67: 785-793. https://doi.org/10.1016/s0165-5876(03)00098-3
- Garcia-Vaquero C, Mir C, Graterol D, et al. Otologic, audiometric and speech findings in patients undergoing surgery for cleft palate. BMC Pediatr 2018; 18: 350. https://doi.org/10.1186/s12887-018-1312-7
- Hubbard TW, Paradise JL, McWilliams BJ, Elster BA, Taylor FH. Consequences of unremitting middle-ear disease in early life. Otologic, audiologic, and developmental findings in children with cleft palate. N Engl J Med 1985; 312: 1529-1534. https://doi.org/10.1056/NEJM198506133122401
- Shaffer AD, Ford MD, Choi SS, Jabbour N. The impact of tympanostomy tubes on speech and language development in children with cleft palate. Otolaryngol Head Neck Surg 2017; 157: 504-514. https://doi.org/10.1177/0194599817703926
- Iemura-Kashiwagi M, Okano T, Iwai N, Taniguchi M, Omori K. Prognosis of otitis media with effusion in pediatric patients with cleft palate during language-acquisition period treated by simultaneous tympanostomy tube placement with palatoplasty. Int J Pediatr Otorhinolaryngol 2022; 155: 111071. https://doi.org/10.1016/j.ijporl.2022.111071
- Ahn JH, Kang WS, Kim JH, Koh KS, Yoon TH. Clinical manifestation and risk factors of children with cleft palate receiving repeated ventilating tube insertions for treatment of recurrent otitis media with effusion. Acta Otolaryngol 2012; 132: 702-707. https://doi.org/10.3109/00016489.2011.652309
- D’Andréa G, Maschi C, Savoldelli C, Caci H, Bailleux S. Otologic outcomes with two different surgical protocols in patients with a cleft palate: a retrospective study. Cleft Palate Craniofac J 2018; 55: 1289-1295. https://doi.org/10.1177/1055665618758686
- Kuo CL, Tsao YH, Cheng HM, et al. Grommets for otitis media with effusion in children with cleft palate: a systematic review. Pediatrics 2014; 134: 983-994. https://doi.org/10.1542/peds.2014-0323
- Møller P. Hearing, middle ear pressure and otopathology in a cleft palate population. Acta Otolaryngol 1981; 92: 521-528. https://doi.org/10.3109/00016488109133291
- Robson AK, Blanshard JD, Jones K, Albery EH, Smith IM, Maw AR. A conservative approach to the management of otitis media with effusion in cleft palate children. J Laryngol Otol 1992; 106: 788-792. https://doi.org/10.1017/s0022215100120894
- Sheahan P, Blayney AW, Sheahan JN, Earley MJ. Sequelae of otitis media with effusion among children with cleft lip and/or cleft palate. Clin Otolaryngol Allied Sci 2002; 27: 494-500. https://doi.org/10.1046/j.1365-2273.2002.00607.x
- Spilsbury K, Ha JF, Semmens JB, Lannigan F. Cholesteatoma in cleft lip and palate: a population-based follow-up study of children after ventilation tubes. Laryngoscope 2013; 123: 2024-2029. https://doi.org/10.1002/lary.23753
- Felton M, Lee JW, Balumuka DD, Arneja JS, Chadha NK. Early placement of ventilation tubes in infants with cleft lip and palate: a systematic review. Otolaryngol Head Neck Surg 2018; 158: 459-464. https://doi.org/10.1177/0194599817742840
- Flynn T, Lohmander A, Moller C, Magnusson L. A longitudinal study of hearing and middle ear status in adolescents with cleft lip and palate. Laryngoscope 2013; 123: 1374-1380. https://doi.org/10.1002/lary.23839
- Jin L, Li K, Li X. Clinical outcomes of otitis media with effusion following palatoplasty in patients with incomplete cleft palate. Acta Otolaryngol 2019; 139: 1-5. https://doi.org/10.1080/00016489.2018.1522449
- Smith TL, DiRuggiero DC, Jones KR. Recovery of eustachian tube function and hearing outcome in patients with cleft palate. Otolaryngol Head Neck Surg 1994; 111: 423-429. https://doi.org/10.1177/019459989411100406
- Veau V, Borel MS. Division palatine: anatomie-chirurgie phonétique. Paris: Masson et Cie; 1931.
- Clark JG. Uses and abuses of hearing loss classification. ASHA 1981; 23: 493-500.
- Gordon AS, Jean-Louis F, Morton RP. Late ear sequelae in cleft palate patients. Int J Pediatr Otorhinolaryngol 1988; 15: 149-156. https://doi.org/10.1016/0165-5876(88)90066-3
- Shaw R, Richardson D, McMahon S. Conservative management of otitis media in cleft palate. J Craniomaxillofac Surg 2003; 31: 316-320. https://doi.org/10.1016/s1010-5182(03)00074-x
- Shaffer AD, Ford MD, Choi SS, Jabbour N. Should children with cleft palate receive early long-term tympanostomy tubes: one institution’s experience. Cleft Palate Craniofac J 2018; 55: 389-395. https://doi.org/10.1177/1055665617736775
- Nomura Y, Oshima H, Nomura K, et al. Outcome of the ‘waiting until spontaneous extrusion’ strategy for long-term tympanostomy tube placement in children with cleft palate. Acta Otolaryngol 2022; 142: 248-253. https://doi.org/10.1080/00016489.2022.2041210
- Schwarz SJ, Brandenburg LS, Weingart JV, et al. Prevalence of tympanostomy tube placement in relation to cleft width and type. Laryngoscope 2021; 131: E2764-E2769. https://doi.org/10.1002/lary.29602
- Yoshitomi A, Baba S, Tamada I, Nakaya M, Itokawa M. Relationship between cleft palate width and otitis media. Laryngoscope Investig Otolaryngol 2022; 7: 2126-2132. https://doi.org/10.1002/lio2.933
- Liu L, Sun YG, Ma L, Zhao W, Wu R. Effect of ventilation tube insertion on otitis media with effusion in cleft palate children. Zhonghua Er Bi Yan Hou Ke Za Zhi 2004; 39: 216-218.
- Li W, Shang W, Yu AH, et al. Early treatment of middle ear disease in cleft palate infants. Hua Xi Kou Qiang Yi Xue Za Zhi 2007; 25: 458-462.
- Greenlund LK, Sajjadi A, Nowariak M, et al. Timing of first set of pressure equalization tubes in pediatric patients with cleft deformities. Laryngoscope 2024; 134: 3391-3394. https://doi.org/10.1002/lary.31285
- Ponduri S, Bradley R, Ellis PE, Brookes ST, Sandy JR, Ness AR. The management of otitis media with early routine insertion of grommets in children with cleft palate - a systematic review. Cleft Palate Craniofac J 2009; 46: 30-38. https://doi.org/10.1597/07-219.1
- Gani B, Kinshuck AJ, Sharma R. A review of hearing loss in cleft palate patients. Int J Otolaryngol 2012; 2012: 548698. https://doi.org/10.1155/2012/548698
- Frisina A, Piacentile K, Frosolini A, Saetti R, Baciliero U, Lovato A. Hearing status and ventilation tube at time of palatoplasty in cleft lip and palate patients: a retrospective study. Medicina (Kaunas) 2023; 59: 513. https://doi.org/10.3390/medicina59030513
- Azman A, Manuel AM. Otological outcome in cleft lip and palate children with middle ear effusion. Int J Pediatr Otorhinolaryngol 2020; 138: 110274. https://doi.org/10.1016/j.ijporl.2020.110274
- Valtonen H, Dietz A, Qvarnberg Y. Long-term clinical, audiologic, and radiologic outcomes in palate cleft children treated with early tympanostomy for otitis media with effusion: a controlled prospective study. Laryngoscope 2005; 115: 1512-1516. https://doi.org/10.1097/01.mlg.0000172207.59888.a2
- Klockars T, Rautio J. Early placement of ventilation tubes in cleft lip and palate patients: does palatal closure affect tube occlusion and short-term outcome? Int J Pediatr Otorhinolaryngol 2012; 76: 1481-1484. https://doi.org/10.1016/j.ijporl.2012.06.028
- Inoue M, Hirama M, Kobayashi S, Ogahara N, Takahashi M, Oridate N. Long-term outcomes in children with and without cleft palate treated with tympanostomy for otitis media with effusion before the age of 2 years. Acta Otolaryngol 2020; 140: 982-989. https://doi.org/10.1080/00016489.2020.1802508
- Maina G, Pollock D, Lockwood C, Cook L, Ooi E. Managing chronic otitis media with effusion in children with non-syndromic cleft palate: short-term ventilation tubes versus surveillance. Cleft Palate Craniofac J 2024; 61: 905-916. https://doi.org/10.1177/10556656221148368
- Alper CM, Losee JE, Seroky JT, Mandel EM, Richert BC, Doyle WJ. Resolution of otitis media with effusion in children with cleft palate followed through five years of age. Cleft Palate Craniofac J 2016; 53: 607-613. https://doi.org/10.1597/15-130
- Gallagher ER, Wu D, Christianson E, et al. Characterization of hearing status in children under 3 years of age with cleft palate. Int J Pediatr Otorhinolaryngol 2022; 162: 111295. https://doi.org/10.1016/j.ijporl.2022.111295
- Smerica AM, Amer R, Edmonds J 3rd, Edmonds JL Jr. Otology and audiology: complications, challenges, and concerns in the patient with cleft lip and/or palate. J Craniofac Surg Forthcoming 2024. https://doi.org/10.1097/SCS.0000000000010396
- Hidaka H, Ito M, Ikeda R, et al. Clinical practice guidelines for the diagnosis and management of otitis media with effusion (OME) in children in Japan - 2022 update. Auris Nasus Larynx 2023; 50: 655-699. https://doi.org/10.1016/j.anl.2022.12.004
- Vlastarakos PV, Nikolopoulos TP, Korres S, Tavoulari E, Tzagaroulakis A, Ferekidis E. Grommets in otitis media with effusion: the most frequent operation in children. But is it associated with significant complications? Eur J Pediatr 2007; 166: 385-391. https://doi.org/10.1007/s00431-006-0367-x
- Ungkanont K, Boonyabut P, Komoltri C, Tanphaichitr A, Vathanophas V. Surveillance of otitis media with effusion in Thai children with cleft palate: cumulative incidence and outcome of the management. Cleft Palate Craniofac J 2018; 55: 590-595. https://doi.org/10.1177/1055665617730361
- Smillie I, Robertson S, Yule A, Wynne DM, Russell CJ. Complications of ventilation tube insertion in children with and without cleft palate: a nested case-control comparison. JAMA Otolaryngol Head Neck Surg 2014; 140: 940-943. https://doi.org/10.1001/jamaoto.2014.1657
- Reiter R, Haase S, Brosch S. Repaired cleft palate and ventilation tubes and their associations with cholesteatoma in children and adults. Cleft Palate Craniofac J 2009; 46: 598-602. https://doi.org/10.1597/08-166.1
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















