Graphical Abstract
Abstract
Background. Infections induced by Shiga toxin-producing Escherichia coli (STEC), especially non-O157 serogroups like O145, pose considerable public health risks. Household transmission is crucial in the dissemination of STEC, particularly in settings characterized by close interaction, such as extended families. This study examines a case of a 5-month-old infant with hemolytic uremic syndrome (HUS) attributed to stx1c-positive STEC and analyzes transmission patterns within the household.
Methods. Perianal swab samples were obtained from a 5-month-old infant diagnosed with STEC-associated HUS and six additional household members. Samples of breast milk were examined as well. Samples were inoculated into sorbitol MacConkey agar (SMAC) and cefixime tellurite sorbitol MacConkey agar (CT-SMAC). Polymerase chain reaction (PCR) was utilized to identify stx1, stx2, and O serogroups. Fecal shedding was investigated over a four-month period with repeated sampling.
Results. Six household members, including the infant, tested positive for stx1, although the mother and breast milk samples were negative. The detected strains were classified within the O145 serogroup and exhibited the stx1c variation. Fecal shedding continued for up to four months in the majority of family members, with the infant exhibiting the briefest length of shedding. The family indicated regular intake of raw meatballs (“çiğköfte”), a traditional Turkish food, made with raw meat, identified as a possible source of illness. None of the family members displayed any symptoms except for the infant, who had severe HUS.
Conclusion. This study underscores the critical impact of household transmission on the dissemination of STEC and the hazards associated with traditional raw meat meals such as çiğköfte. Non-O157 STEC serogroups, including O145, are increasingly recognized as significant agents of human infections. The results underscore the significance of monitoring, hygiene education, and preventive strategies to mitigate the dissemination of STEC in families and the wider community. Mitigating extended fecal shedding and detecting foodborne transmission sources are essential for effective public health intervention.
Keywords: hemolytic uremic syndrome, shiga toxin-producing Escherichia coli, STEC O145, stx1c
Introduction
The dissemination of Shiga toxin-producing Escherichia coli (STEC) within households presents a considerable public health threat owing to its potential to cause severe illness and its ability for fast transmission in enclosed environments. Studies indicate that home transmission rates may differ, with some showing rates ranging from 4% to 15% after isolated infections.1 Young children are especially susceptible, serving both as carriers and as potential sources for increased community transmission.1,2
Dynamics of transmission for STEC are influenced by the particular strain in question. For example, in the 2011 outbreak in Germany, the STEC O104:H4 strain demonstrated prolonged shedding in specific carriers, despite the rarity of secondary transmission within households.3 This underscores the necessity of understanding the unique characteristics of the STEC strain involved in an outbreak to develop appropriate public health policies.
STEC can spread through various routes, with foodborne transmission recognized as the primary route. The consumption of undercooked or raw meat, especially beef, significantly contributes to the occurrence of STEC infections. Engaging directly with animals, especially ruminants like cattle, which act as key reservoirs, represents a significant route of transmission.4 Transmission from one individual to another takes place within homes or communities, especially impacting children younger than five years, who are more susceptible to disseminating the infection.1,4 Furthermore, STEC can endure in various environments such as soil, water, and agricultural runoff, resulting in indirect transmission by contact with contaminated surfaces or water sources.5
The transmission of STEC within households poses a complex challenge necessitating a comprehensive approach, which includes timely detection, case isolation, and focused hygiene education, especially in homes with young children.6,7 Family clusters of STEC infections underscore the necessity of broadening epidemiological investigations to include all household members, given that the initial case may not consistently be the primary source of infection.8 Comprehending these dynamics is essential for developing effective public health strategies to control and prevent STEC outbreaks, both within households as well as the wider community.
This study examines the significance of household transmission of STEC through the case of a 5-month-old infant, who was exclusively breastfed and diagnosed with STEC-related hemolytic uremic syndrome (HUS). Additionally, we investigated the extended family living with the infant for STEC carriage.
Materials and Methods
Case description
A previously healthy 5-month-old infant, was brought to the hospital by the family due to vomiting and diarrhea persisting for 4 days. The physical examination showed signs of dehydration. The peripheral blood smear revealed fragmented red blood cells (helmet cells). The patient, who presented with diarrhea, thrombocytopenia, anemia, and impaired kidney function tests, was admitted to the pediatric department with a diagnosis of HUS. The diagnostic criteria for HUS included hemolytic anemia with a hemoglobin (Hb) level of <10 g/dL, thrombocytopenia (platelets <150,000/μL) and acute renal injury (serum creatinine ≥1.5 times the upper limit of normal).9 A Doppler ultrasound of the urinary system showed mild increased echogenicity in the renal parenchyme. A perianal swab sample was cultured, and polymerase chain reaction (PCR) testing for STEC was performed on the bacteria that grew in the culture. The patient tested positive for stx1 and was followed up in the department. During the 10-day hospital stay, the patient experienced vomiting attacks 4-5 times a day, watery diarrhea 7-8 times, anemia, leukocytosis, thrombocytopenia, oliguria, macroalbuminuria, and signs of acute renal failure. During hospitalization, the patient did not require dialysis but needed erythrocyte suspensions. No antibiotics were administered to the patient, who had no history of antibiotic use before hospitalization. The family lives in Kocaeli city center, Türkiye, and has no history of animal husbandry. Since the infant was exclusively breastfed and there was no food source that could transmit STEC, household transmission was suspected. Upon the family’s approval to investigate STEC carriage, perianal swab samples were collected from individuals living in the same household.
Study plan
This study was conducted by the Department of Medical Microbiology, Faculty of Medicine, Kocaeli University. The study was conducted with the approval of the Clinical Research Ethics Committee and informed consent forms were obtained from each participant prior to enrollment. All procedures were conducted in accordance with the ethical standards of the Declaration of Helsinki.
Sample collection, culture and DNA isolation
Perianal swab samples were taken from the 5-month-old infant diagnosed with HUS and from six individuals living in the same household. The family was an extended family consisting of the index case (5m), mother (21y), father (25y), grandmother (47y), grandfather (45y), uncle (21y), and aunt (13y), living in the same house. Additionally, a sample of breast milk was collected. The perianal swab samples were transported in a Stuart transport medium, while the breast milk sample was delivered to the laboratory in a dry tube. Samples were taken from individuals one week after the initial detection and once a month for four months. Sorbitol MacConkey (SMAC) agar and cefixime sellurite sorbitol MacConkey (CT-SMAC) agar were used for inoculating samples. The plates were incubated at 37 °C in the incubator for 24 hours. If no growth was observed, the incubation time was extended to 48 hours. STEC suspicious colonies were selected and transferred on SMAC agar and incubated at 37°C in the incubator for 24 hours. The boiling extraction method was used for DNA isolation. The NanoDrop spectrophotometer was used for DNA quantification. After DNA isolation, stx positive isolates were identified by PCR method.
Determination of stx genes, stx variants and O serogroups
stx1 and stx2 genes were investigated by the conventional PCR method.10 O26, O45, O103, O104, O111, O121, O145 and O157 gene regions were investigated by the conventional PCR method according to the study by Paddock et al.11 Stx1 variants (Stx1a, Stx1c and Stx1d) were investigated by conventional PCR. The primers of the Stx1 variants, the mixture prepared for the reaction, and the temperature cycles were referenced according to the study by Scheutz et al.12
Results
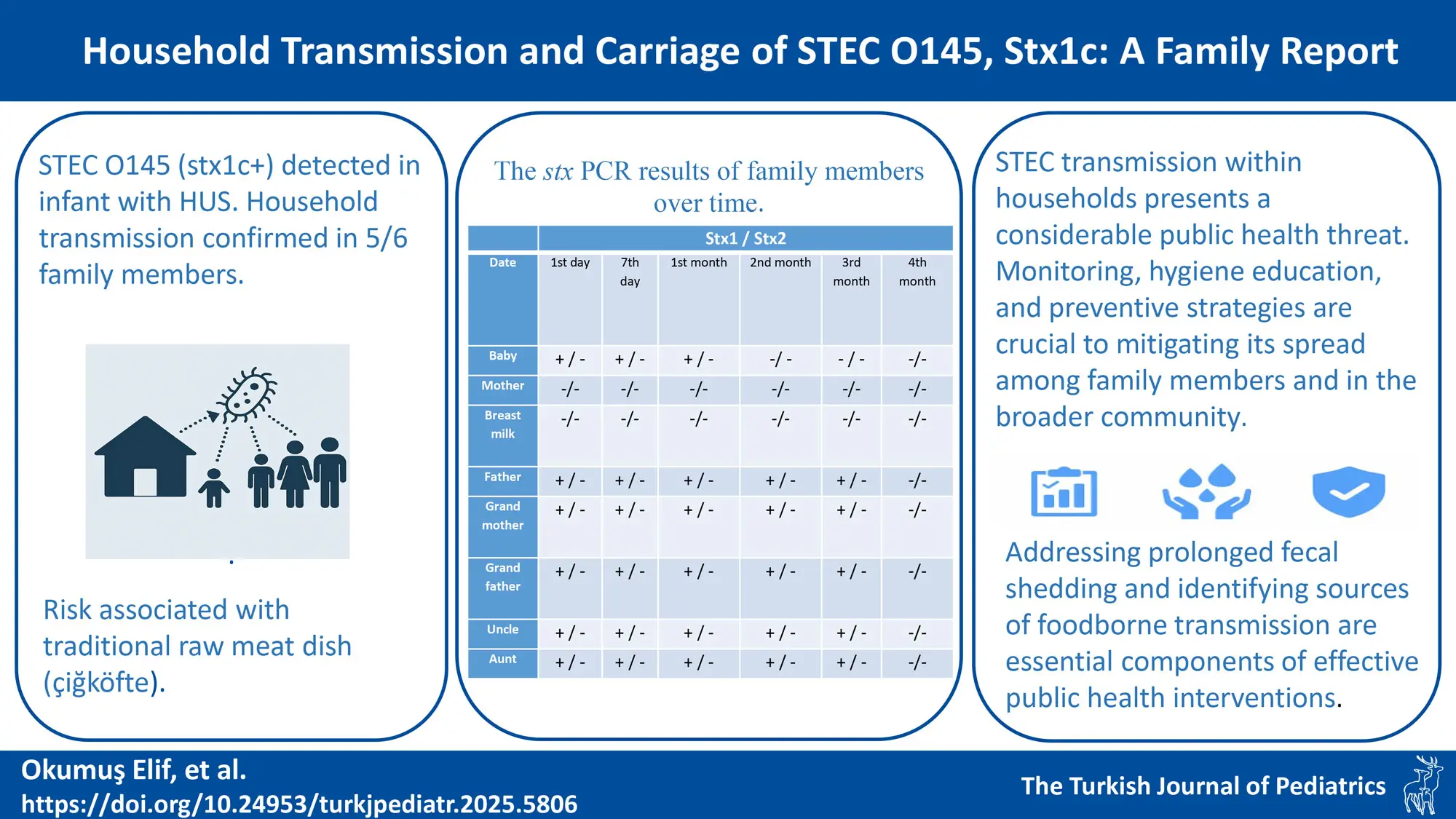
Perianal swab samples taken from the infant and six individuals living in the same household were screened for stx1 and stx2 using PCR. All family members excluding the mother tested positive for stx1. The breast milk sample taken at the time of admission was found to be negative for stx-PCR. Perianal swab samples were taken from the individuals on the 7th day, 1st month, 2nd month, 3rd month, and 4th month after hospital admission for carrier surveillance. The stx PCR results of family members over time are summarized in Table I. The strains were investigated for the O serogroup and toxin variants, and all strains were found to be positive for the O145 serogroup and the stx1c variant.
Multiple interviews were conducted with the family to investigate the source of the STEC infection. The 5-month-old patient had been exclusively breastfed and had not received any complementary foods. The mother reported not consuming meat, as she follows a vegetarian diet. Other family members, however, regularly consumed meat products. None of the family members had experienced any clinical symptoms suggestive of STEC infection in the two weeks prior to presentation. The family reported consuming a traditional Turkish dish made with raw meat (“çiğköfte”) eight days before presentation and noted that they frequently consume çiğköfte. They suggested that this food might have been the source of the STEC infection.
Discussion
Household transmission of STEC is a major concern because of its rapid spread between individuals and the potential severity of the infection. In households, children are particularly vulnerable, both to acquiring and transmitting the infection.7 Studies have indicated that siblings and mothers are at a higher risk of contracting the infection from an infected child, with transmission rates in households varying between 0% and 34.4%.1,6 In this study, the infant diagnosed with HUS had an extended family and the family members were very young. The detection of STEC in all household members except the mother suggests that this case was a result of household transmission. Although the infant had no siblings, the aunt, who was living in the same house, was a 13-year-old child. The infant was exclusively breastfed. Although there is no data in the literature on STEC transmission through breast milk, samples were also taken from breast milk because breastmilk was the infant’s only source of nutrition. The infant’s close contacts were the mother and the child’s aunt. Since the mother was negative for stx and transmission among children is more common, we suspected that the transmission occurred from the aunt. Although adults can acquire the infection, transmission rate is generally lower than that observed in children.8 Transmission of STEC within families is significantly influenced by closeness and hygiene practices, with children playing an important part in disseminating the illness. Preventive measures, such as isolating sick individuals and promoting hygiene education, are essential for reducing transmission rates, especially among young children.6,13 For example, promptly separating siblings after a diagnosis has been recommended as an effective approach to limit further transmission.2
In the study on the outbreak of O26:H11 STEC by Brown et al., the risk of infection in children <36 months was twice the risk among children of 36 to 47 months.14 Although STEC was detected in all family members in the study, only the infant developed HUS. We suggest the reason for that is that younger children have a less developed immune response, making them more susceptible to infections and potential complications like HUS.14-16 In a study by Alconcher et al.7 including 82 HUS patients, 36.6% of HUS patients had 36 STEC-positive household contacts and nearly one third of them were children. There was a high concordance (83%) between the serotype and/or stx-genotype of HUS patients and their household contacts.7 Similarly, sxt1c and O145 serogroups were detected in all family members included in this study.
The family stated that çiğköfte could be the source of STEC infection. Çiğköfte is a traditional Turkish street food often made with raw meat, posing potential health risks due to contamination with STEC. Research has shown that STEC, particularly E. coli O157, might be present in çiğköfte. The research in Türkiye found that E. coli O157 was detected in 20.8% of meat-based and 14.6% of vegetarian çiğköfte samples.17 This highlights the possible risk of STEC infection in both meat and vegetarian versions of the food.
While STEC O157:H7 was previously considered the most common serotype in HUS patients, the recognition of non-O157 STEC isolates has been increasing in recent years. The STEC serogroups O26, O45, O103, O111, O121, O145 and O157 represent the “top seven” STEC serogroups that are common in humans.18,19 For this reason, this study conducted a PCR analysis encompassing the seven predominant serogroups for O serogroup detection. In the study by Carbonari et al.20, STEC O145 was the second most common serogroup associated with HUS, following O157 and it accounted for 20.3% of HUS cases in Argentina. STEC has several variants and epidemiologic studies suggest that Stx2 variants differ in potency and cause different clinical conditions.21 Stx1c-producing STEC strains are often eae-negative and belong to various serotypes not typically associated with severe human disease, such as O157, O26, O103, O111, or O145.22,23 Stx1c and O145 serogroups were detected in the family members included in the study. Stx1c and O145 STEC strains are important contributors to human infections, with distinct genetic and virulence profiles. While stx1c is often associated with milder symptoms, O145 can cause severe disease and is a concern in outbreak scenarios.23,24 Understanding their prevalence, identification, and transmission patterns is essential for reducing public health risks associated with these diseases.
Studies have shown that fecal shedding can be both extended and sporadic. A study conducted among children in Argentina revealed that distinct STEC strains were shed for durations ranging from 19 to 37 days, highlighting the variability in shedding duration among different serotypes.25 In the 2011 outbreak in Germany, the median period of pathogen shedding was seen to be 17-18 days, with certain patients shedding the virus for as long as 157 days.26 Prolonged fecal shedding of STEC was noted in the family members participating in the study. The minimal shedding duration was noted in the infant with HUS. Fecal shedding of STEC may be reduced in patients with HUS relative to those without HUS, and therapy can affect the duration of shedding.26,27 The extended shedding of STEC has considerable consequences for managing the dissemination of infection. Infected persons, particularly in high-risk settings such as childcare facilities, may require exclusion until they test negative for STEC to prevent further cases.25,28
The limitation of the study is that clonal relationships between isolates could not be examined. Investigating clonal relationships and increasing the study populations are among the future goals of the researchers.
Conclusion
STEC poses a significant public health challenge, particularly in environments where close contact among family members facilitates pathogen spread. This study highlights the transmission dynamics of STEC within a household, emphasizing the role of young children in the dissemination of the infection. The detection of the same STEC strain in multiple family members, except for the mother, strongly suggests intra-household transmission as the primary route of infection. The prolonged fecal shedding observed in family members further illustrates the potential for extended transmission within households, reinforcing the need for strict hygiene measures and early detection strategies.
Ethical approval
The study was approved by Kocaeli University Non-Interventional Clinical Research Ethics Committee (date: 08.05.2019, number: 2019/289).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Parry SM, Salmon RL. Sporadic STEC O157 infection: secondary household transmission in Wales. Emerg Infect Dis 1998; 4: 657-661. https://doi.org/10.3201/eid0404.980419
- Werber D, Mason BW, Evans MR, Salmon RL. Preventing household transmission of Shiga toxin-producing Escherichia coli O157 infection: promptly separating siblings might be the key. Clin Infect Dis 2008; 46: 1189-1196. https://doi.org/10.1086/587670
- Sin MA, Takla A, Flieger A, et al. Carrier prevalence, secondary household transmission, and long-term shedding in 2 districts during the Escherichia coli O104:H4 outbreak in Germany, 2011. J Infect Dis 2013; 207: 432-438. https://doi.org/10.1093/infdis/jis702
- Kintz E, Brainard J, Hooper L, Hunter P. Transmission pathways for sporadic Shiga-toxin producing E. coli infections: a systematic review and meta-analysis. Int J Hyg Environ Health 2017; 220: 57-67. https://doi.org/10.1016/j.ijheh.2016.10.011
- Fremaux B, Prigent-Combaret C, Vernozy-Rozand C. Long-term survival of Shiga toxin-producing Escherichia coli in cattle effluents and environment: an updated review. Vet Microbiol 2008; 132: 1-18. https://doi.org/10.1016/j.vetmic.2008.05.015
- Tokuda K, Yahata Y, Sunagawa T. Prevention of secondary household transmission during Shiga toxin-producing Escherichia coli outbreaks. Epidemiol Infect 2016; 144: 2931-2939. https://doi.org/10.1017/S0950268816001199
- Alconcher LF, Rivas M, Lucarelli LI, Galavotti J, Rizzo M. Shiga toxin-producing Escherichia coli in household members of children with hemolytic uremic syndrome. Eur J Clin Microbiol Infect Dis 2020; 39: 427-432. https://doi.org/10.1007/s10096-019-03738-1
- Luini MV, Colombo R, Dodaro A, et al. Family clusters of Shiga toxin-producing Escherichia coli infection: an overlooked source of transmission. Data from the ItalKid-Hus network. Pediatr Infect Dis J 2021; 40: 1-5. https://doi.org/10.1097/INF.0000000000002877
- Hirata C, Kenzaka T, Akita H. Late onset of hemolytic uremic syndrome after the appearance of prodromal gastrointestinal tract symptoms. Clin Case Rep 2020; 8: 1910-1913. https://doi.org/10.1002/ccr3.3020
- Cebula TA, Payne WL, Feng P. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J Clin Microbiol 1995; 33: 248-250. https://doi.org/10.1128/jcm.33.1.248-250.1995
- Paddock Z, Shi X, Bai J, Nagaraja TG. Applicability of a multiplex PCR to detect O26, O45, O103, O111, O121, O145, and O157 serogroups of Escherichia coli in cattle feces. Vet Microbiol 2012; 156: 381-388. https://doi.org/10.1016/j.vetmic.2011.11.017
- Scheutz F, Teel LD, Beutin L, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol 2012; 50: 2951-2963. https://doi.org/10.1128/JCM.00860-12
- Pereboom M, Todkill D, Knapper E, Jenkins C, Hawker J, Coetzee N. Shiga toxin-producing Escherichia coli (STEC) O157 outbreak associated with likely transmission in an inflatable home paddling pool in England, June 2017. Perspect Public Health 2018; 138: 279-281. https://doi.org/10.1177/1757913918774072
- Brown JA, Hite DS, Gillim-Ross LA, et al. Outbreak of shiga toxin-producing Escherichia coli serotype O26: H11 infection at a child care center in Colorado. Pediatr Infect Dis J 2012; 31: 379-383. https://doi.org/10.1097/INF.0b013e3182457122
- Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 2017; 46: 350-363. https://doi.org/10.1016/j.immuni.2017.03.009
- McKee RS, Schnadower D, Tarr PI, et al. Predicting hemolytic uremic syndrome and renal replacement therapy in shiga toxin-producing Escherichia coli-infected children. Clin Infect Dis 2020; 70: 1643-1651. https://doi.org/10.1093/cid/ciz432
- Ghazzi M, Porto-Fett A, Ayaz N, et al. Microbiological characterization of çiğ köfte sold at retail in Ankara, Turkey, and evaluation of selected antimicrobials as ingredients to control foodborne pathogens in çiğ köfte during refrigerated storage. Food Control 2018; 84: 138-147. https://doi.org/10.1016/j.foodcont.2017.04.033
- Bosilevac JM, Koohmaraie M. Prevalence and characterization of non-O157 shiga toxin-producing Escherichia coli isolates from commercial ground beef in the United States. Appl Environ Microbiol 2011; 77: 2103-2112. https://doi.org/10.1128/AEM.02833-10
- Cukrovany AE, Wroblewski D, Wirth SE, et al. Shiga toxin-producing Escherichia coli testing in New York 2011-2022 reveals increase in Non-O157 Identifications. Foodborne Pathog Dis 2025; 22: 273-280. https://doi.org/10.1089/fpd.2023.0152
- Carbonari, CC, Miliwebsky, ES, Zolezzi, G., et al. (2022). The importance of shiga toxin-producing Escherichia coli O145:NM[H28]/H28 infections in Argentina, 1998–2020. Microorganisms 10, 582. https://doi.org/10.3390/microorganisms10030582
- Fuller CA, Pellino CA, Flagler MJ, Strasser JE, Weiss AA. Shiga toxin subtypes display dramatic differences in potency. Infect Immun 2011; 79: 1329-1337. https://doi.org/10.1128/IAI.01182-10
- Friedrich AW, Borell J, Bielaszewska M, Fruth A, Tschäpe H, Karch H. Shiga toxin 1c-producing Escherichia coli strains: phenotypic and genetic characterization and association with human disease. J Clin Microbiol 2003; 41: 2448-2453. https://doi.org/10.1128/JCM.41.6.2448-2453.2003
- Zhang W, Bielaszewska M, Kuczius T, Karch H. Identification, characterization, and distribution of a Shiga toxin 1 gene variant (stx(1c)) in Escherichia coli strains isolated from humans. J Clin Microbiol 2002; 40: 1441-1446. https://doi.org/10.1128/JCM.40.4.1441-1446.2002
- Wahl E, Vold L, Lindstedt BA, Bruheim T, Afset JE. Investigation of an Escherichia coli O145 outbreak in a child day-care centre-extensive sampling and characterization of eae- and stx1-positive E. coli yields epidemiological and socioeconomic insight. BMC Infect Dis 2011; 11: 238. https://doi.org/10.1186/1471-2334-11-238
- Miliwebsky E, Deza N, Chinen I, et al. Prolonged fecal shedding of Shiga toxin-producing Escherichia coli among children attending day-care centers in Argentina. Rev Argent Microbiol 2007; 39: 90-92.
- Vonberg RP, Höhle M, Aepfelbacher M, et al. Duration of fecal shedding of Shiga toxin-producing Escherichia coli O104:H4 in patients infected during the 2011 outbreak in Germany: a multicenter study. Clin Infect Dis 2013; 56: 1132-1140. https://doi.org/10.1093/cid/cis1218
- Nitschke M, Sayk F, Härtel C, et al. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA 2012; 307: 1046-1052. https://doi.org/10.1001/jama.2012.264
- Mody RK, Griffin PM. Fecal shedding of Shiga toxin-producing Escherichia coli: what should be done to prevent secondary cases? Clin Infect Dis 2013; 56: 1141-1144. https://doi.org/10.1093/cid/cis1222
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















