Graphical Abstract
Abstract
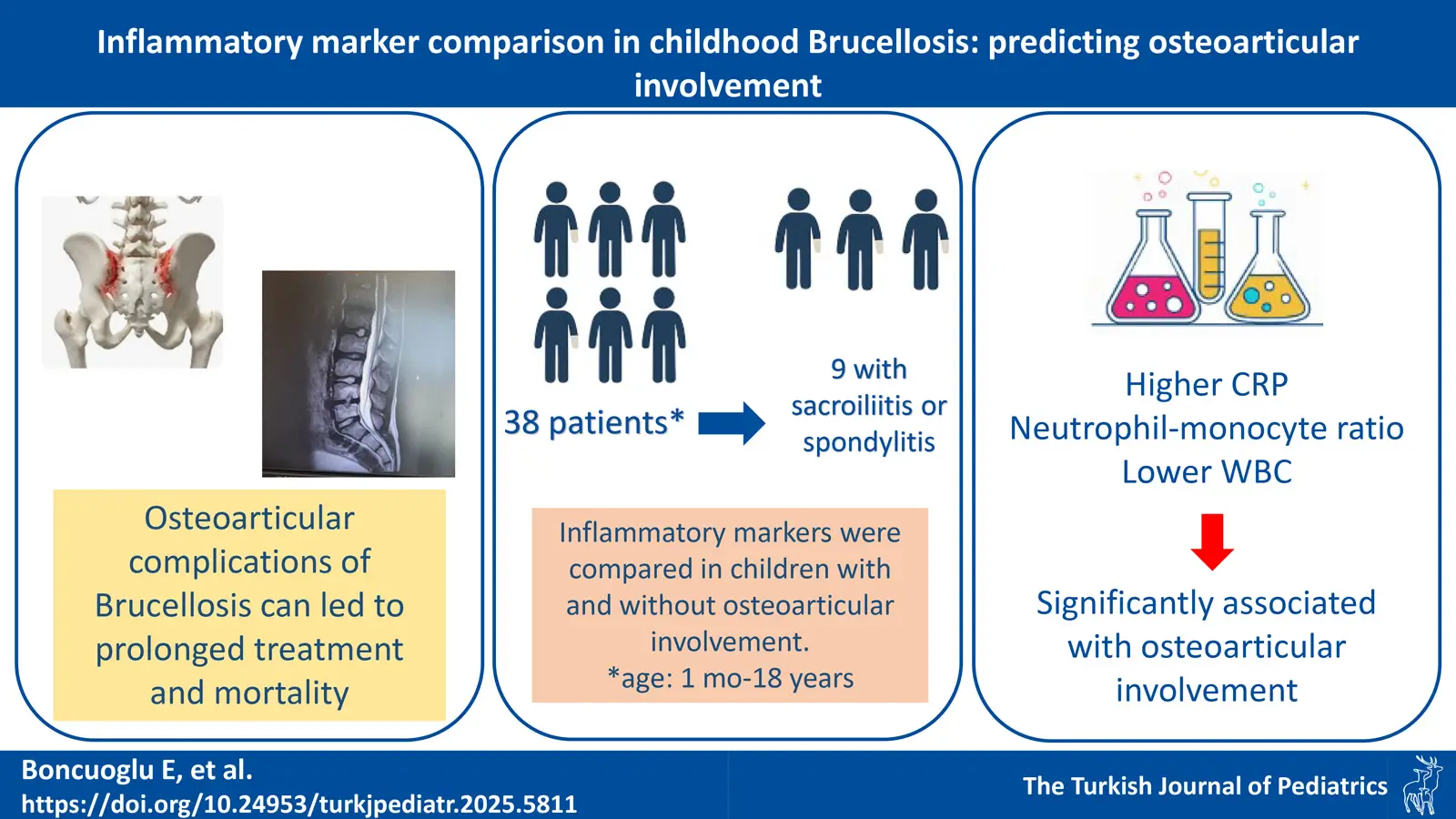
Background. Although the use of inflammatory markers in diagnosing Brucella-related complications has been the subject of research, studies on osteoarticular disease are insufficient, especially in children. This study aimed to compare inflammatory markers in children diagnosed with brucellosis, distinguishing between those with and without osteoarticular involvement (OI).
Methods. In this retrospective study, patients diagnosed with brucellosis from 1 month to 18 years of age were evaluated. Data collected included age, gender, OI, treatment duration, complete blood count, inflammatory markers including neutrophil-monocyte ratio (NMR), monocyte-lymphocyte ratio, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and Brucella serum agglutination test (SAT) results. OI was confirmed by MRI in symptomatic patients. The results of patients with and without OI were compared.
Results. The study included 38 patients, 23.7% having OI (8 with sacroiliitis and 1 with spondylitis). The median age of patients with OI was significantly higher than those without (p=0.037). All patients with OI (n = 9, 100%) had an SAT titer ≥ 1/640. Among patients without OI, 62% (n = 18) had an SAT titer ≥1/640. This difference was statistically significant (p = 0.028). Patients with OI had higher CRP levels (p=0.038) but similar ESR levels compared to those without. WBC levels were significantly lower in the group with OI (p=0.015). NMR was significantly higher in those with OI (p=0.012).
Conclusions. Lower WBC counts and higher CRP and NMR levels can predict OI in children with brucellosis at the time of admission. However, our findings should be validated through prospective studies involving larger patient groups.
Keywords: brucellosis, osteoarticular brucellosis, neutrophile-to-monocyte ratio
Introduction
Brucellosis is one of the most prevalent zoonoses, causing some serious public health consequences.1 Globally, approximately 500,000 human cases of brucellosis are reported annually.2 According to European Center for Disease Prevention and Control (ECDC) data, the notification rate in Europe was 0.04 cases per 100,000 population.3 Brucellosis is also endemic in Türkiye, with widespread disease throughout the country; however, the regions with the highest incidence are the southeast and east parts of the country. The Turkish Ministry of Health reported the incidence of brucellosis as 12.3 per 100,000 in 2019.4
Osteoarticular involvement (OI) is a widely reported manifestation in adults and is also the primary complication of brucellosis in children.5,6 Brucellosis can lead to prolonged fever, hemophagocytic lymphohistiocytosis, endocarditis, meningitis, as well as peripheral arthritis, osteomyelitis, and, less commonly, severe osteoarticular complications such as spondylitis and sacroiliitis.7 These complications in children not only cause school failure and economic burden due to hospitalization and long-term antibiotic use but also cause psychological problems along with loss of workforce, especially in adolescents. Therefore, early recognition of complications is essential.
Hematological parameters, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) are easily accessible markers commonly used in the follow-up of infections. Although the use of inflammatory markers in diagnosing brucellosis complications is a subject of research, studies on OI and the use of these markers to manage the disease remain limited, especially in children. This study aims to compare the inflammatory markers between patients with and without osteoarticular involvement.
Materials and Methods
The medical records of 48 patients (age range: 1 month to 18 years) diagnosed with brucellosis at the Department of Pediatrics and Pediatric Infectious Diseases in Konya City Hospital between July 2022 and January 2024 were retrospectively reviewed. After excluding the patients with incomplete demographic information, test results, or those transferred to another hospital for follow-up and treatment, the remaining 38 patients were enrolled. The following data were recorded for the patients: age, gender, presence of OI, presence of fever, treatment duration, complete blood count (including leukocyte, lymphocyte, neutrophil, monocyte, and platelet counts, mean platelet volume), inflammatory markers [neutrophil-to-monocyte ratio (NMR), monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), CRP, and ESR], and Brucella serum agglutination test (SAT) results at the time of admission. Serum samples were incubated with Brucella abortus antigen (Brucella abortus tube antigen supplied by the Public Health Institution of Türkiye, Ankara, Türkiye). Brucella antigen was evaluated for agglutinin particles for 24 h at 37°C, and samples with ≥ 1/160 were considered positive.
Definitions
OI was confirmed by magnetic resonance imaging (MRI) in patients with symptoms. Symptoms indicative of sacroiliitis included hip pain, inability to walk, and restriction of movement. Similarly, spinal pain, tenderness along the paravertebral region, and restricted movement were considered as symptoms of spondylitis.
The inflammatory markers of patients with and without OI were compared.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (version 29.0.2.0; SPSS). Categorical variables were analyzed using relative frequencies, whereas numerical variables were analyzed using median or mean values (depending on whether they had a normal distribution). Categorical variables were compared using Pearson χ2 and Fisher’s exact tests. Numerical variables were compared using the t-test or the nonparametric Mann–Whitney U test. A p < 0.05 was considered to be statistically significant.
Ethical approval
The study was approved by the Necmettin Erbakan University Ethics Committee (date: 05.07.2024, number: 2024/5064)
Results
A total of 38 patients diagnosed with brucellosis were included in the study. Of the patients 65.8% were male and 34.2% female. The median age of the patients was 13 years (IQR: 9-15). Upon admission, 47.4% of the patients (18/38) presented with a fever. OI was proven by MRI in 23.7% (9/38) of the patients (sacroiliitis in 8 patients and spondylitis in 1 patient), while the other patients had no organ involvement. When comparing patients with and without OI, no significant gender difference was found (p=0.95). However, the mean age of patients with OI was significantly higher than those without (p=0.004).
From a diagnostic perspective, SAT titers ranged from 1/160 to 1/5120 among all patients. All patients with OI (n = 9, 100%) had an SAT titer ≥ 1/640. Among patients without OI, 62% (n = 18) had an SAT titer ≥1/640. This difference was statistically significant (p = 0.028). When comparing treatment durations, patients with OI received treatment significantly longer (median 6 weeks vs. 10,5 weeks, p=0.002).
Regarding symptoms and laboratory findings suggestive of an inflammatory process, there was no significant difference in fever between the two groups (p=0.18). The level of CRP was higher in patients with OI (p=0.039), while ESR was similar between the groups. Regarding hematologic parameters, white blood cell (WBC) count was significantly lower in the group with OI (p=0.013). Although the absolute lymphocyte, neutrophil, and monocyte counts were similar, NMR was significantly higher in the group with OI (p=0.012). The other markers, MLR, NLR, PLR, and MPV, were similar between the groups (Table I).
|
*mean ± SD (min-max), ** median (Q1-Q3) ALC, Absolute lymphocyte count; AMC, Absolute monocyte count; ANC, Absolute neutrophile count; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; MLR, Monocyte-to-lymphocyte ratio; MPV, Mean corpuscular volume; NLR, Neutrophile-to-lymphocyte ratio; NMR, Neutrophile-to-monocyte ratio; PLR, Platelet-to-lymphocyte ratio; PLT, Platelet count; SAT, Serum agglutination test; WBC, White blood cell count. |
|||
| Table I. Demographics and laboratory findings of the patients (N=38). | |||
| Patients with osteoarticular involvement | Patients without osteoarticular involvement | p-value | |
| Number of patients (%) | 9 (23.7%) | 29 (76.3%) | |
| Age (year)* | 14.6 ±2.2 (10-17) | 11.1 ±4.4 (3-17) | 0.004 |
| Gender, n (%) | 0.950 | ||
| Male | 6 (66.7%) | 19 (65.5%) | |
| Female | 3 (33.3%) | 10 (34.5%) | |
| Treatment duration (week)** | 11 (9-12) | 6 (6-7) | 0.002 |
| SAT titer ≥1/640 (%) | 100% | 62% | 0.028 |
| WBC (/µL)** | 5960 (5380-7390) | 7480 (6485-9660) | 0.013 |
| ANC (/µL)** | 2770 (2270-3900) | 3570(2645-4740) | 0.080 |
| ALC (/µL)* | 2478±663 (1170-3530) | 3056 ±1374 (1040-6440) | 0.235 |
| AMC (/µL)** | 500 (470-800) | 550 (425-735) | 0.893 |
| NMR** | 0.2 (0.2-0.2) | 0.1 (0.1-0.2) | 0.010 |
| MLR* | 0.2±0.1 (0.2-0.3) | 0.2±0.2 (0.1-1) | 0.786 |
| NLR* | 1.1±0.5 (0.3-1.9) | 1.7±1.8 (0.1-8.7) | 0.303 |
| PLT (/µL)* | 286888±63142 (193000-381000) | 282586±92422 (78000-456000) | 0.897 |
| PLR** | 120.9 (96.6-137.5) | 101.4 (68.6-120.6) | 0.277 |
| MPV (fL)* | 9.2±0.5 (8.5-9.9) | 9.9±0.9 (8.3-11.8) | 0.064 |
| ESR (mm/h)** | 29 (20-45) | 17 (8-35) | 0.150 |
| CRP (mg/L)** | 30.2 (22.2-55) | 9.8 ((0.9-32.3) | 0.039 |
Discussion
This study indicates that the readily accessible and cost-effective inflammatory markers CRP, WBC, and NMR can serve as indicators of OI in brucellosis. In previous studies, peripheral arthritis was predominantly observed in the pediatric population, whereas sacroiliitis and spondylitis were more frequently identified in adolescents and adults.8,9 Our study also revealed that patients with OI were older than the others, suggesting the potential utility of these markers, particularly in the adolescent age group. Some studies have indicated that OI in childhood brucellosis is more prevalent among males9,10, while others have suggested a higher incidence in females.11,12 In our study, no significant gender difference was observed between patients with and without OI. In the group with OI, the SAT titer was ≥1/640 at diagnosis, and it was statistically different from the group without OI. Although the relationship between SAT titers and disease severity or the presence of complications has not been established, our findings were consistent with those in the study by Çiftdoğan et al. regarding the serologic test results.13
Moreover, in the presence of osteoarticular complications, the literature indicates a recommendation for a combined antibiotic treatment regimen lasting 3-6 months to achieve a complete cure and prevent relapse.14,15 In our study, the treatment duration for patients with osteoarticular disease was significantly longer due to delayed radiological and clinical improvement. It is noteworthy that adolescents may be at risk of experiencing antibiotic side effects due to prolonged treatment.
Kayaaslan and colleagues, in their study involving 700 adult patients, found that CRP and ESR values were significantly elevated in those with hepatitis, epididymoorchitis, neurobrucellosis, and OI.16 A similar multicenter study in China also demonstrated that increased ESR and CRP levels were associated with complicated brucellosis.17Research on pediatric patients with osteoarticular complications supports these findings in adults.13,18 In our study, CRP levels were significantly higher in the group with OI. However, there was no statistically significant difference between the groups concerning ESR elevation. This discrepancy might be because the study was based on blood test results taken at the time of admission, with CRP rising faster than ESR.19 For more accurate disease monitoring, serial measurements of ESR might be necessary. Upon admission, evaluating CRP levels in patients presenting with joint pain at admission could provide more accurate information regarding OI.
Several mechanisms may explain the hematological changes seen in brucellosis, such as hypersplenism, bone marrow suppression, direct bacterial infection of hematologic cells, and immune-mediated destruction, which can result in leukopenia, anemia, and thrombocytopenia.20 Our study found that the mean WBC was lower in patients with OI, although the groups had similar thrombocyte levels. Recent research highlights that LMR, NLR, and PLR are valuable markers of systemic inflammation in various diseases.21-23 Olt et al. reported a significant decrease in NLR in brucellosis patients compared to healthy individuals and a higher median NLR in those with arthritis.24 Another study observed significantly elevated PLR and NLR in children with Brucella arthritis.25 In our study, only the NMR, which has been shown to be beneficial in sepsis, COVID-19, and non-infectious inflammation, was significantly higher in patients with OI.26-28 To the best of our knowledge, this is the first study evaluating NMR in brucellosis. MPV has also been identified as an inflammation marker in many diseases, with two studies indicating higher MPV in children with arthritis-positive brucellosis compared to healthy controls and those with arthritis-negative brucellosis.21,25,29 Sen et al. found lower MPV in brucellosis patients with specific organ involvement versus those with uncomplicated brucellosis. In our study, mean MPV was lower in the OI group, but this difference was not statistically significant.30
Our study has several limitations. First, since our study is retrospective, we could only evaluate the blood tests taken at the time of the patients’ initial presentation. Changes in inflammatory markers during the follow-up could not be observed. Second, the design of the study does not include a control group. Third, due to the retrospective nature of the study, the patients’ symptoms and the duration of these symptoms could not be analyzed in detail. Consequently, the relationship between the stage of infection (acute, subacute, or chronic) and inflammatory markers could not be evaluated.
In conclusion, lower WBC and higher CRP and NMR levels can serve as indicators for predicting OI at the time of admission. These readily accessible markers could assist clinicians in the early identification of complications, facilitating timely intervention. Prospective studies with larger cohorts and serial marker evaluations are required to validate and expand on our results, improving the accuracy and reliability of inflammatory markers in diagnosing and managing osteoarticular complications in pediatric brucellosis.
Ethical approval
The study was approved by the Necmettin Erbakan University Ethics Committee (date: 05.07.2024, number: 2024/5064).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- World Health Organization (WHO). Brucellosis. Available at: https://www.who.int/news-room/fact-sheets/detail/brucellosis (Accessed on January 19, 2025).
- Negron M, Tiller R, Kharod G. Brucellosis. In: CDC Yellow Book 2024: Health Information for International Travel. 2023. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/brucellosis#epi (Accessed on March 23, 2025).
- European Centre for Disease Prevention and Control. Brucellosis. In: ECDC. Annual epidemiological report for 2021. Stockholm: ECDC; 2023.
- Republic of Türkiye Ministry of Health. Brucellosis statistical data. Available at: https://hsgm.saglik.gov.tr/depo/birimler/zoonotik-ve-vektorel-hastaliklar-db/Dokumanlar/Raporlar/Tr_Bruselloz_Mevcut_Durum.pdf (Accessed on March 23, 2025).
- Shaalan MA, Memish ZA, Mahmoud SA, et al. Brucellosis in children: clinical observations in 115 cases. Int J Infect Dis 2002; 6: 182-186. https://doi.org/10.1016/s1201-9712(02)90108-6
- Gür A, Geyik MF, Dikici B, et al. Complications of brucellosis in different age groups: a study of 283 cases in southeastern Anatolia of Turkey. Yonsei Med J 2003; 44: 33-44. https://doi.org/10.3349/ymj.2003.44.1.33
- Young EJ. Brucella species (brucellosis). In: Long SS, Prober CG, Fischer M, editors. Principles and practice of pediatric infectious diseases. 6th ed. Chapter 161. Elsevier; 2023. https://doi.org/10.1016/B978-0-323-75608-2.00161-0
- Hizel K, Guzel O, Dizbay M, et al. Age and duration of disease as factors affecting clinical findings and sacroiliitis in brucellosis. Infection 2007; 35: 434-437. https://doi.org/10.1007/s15010-007-6361-z
- Zamani A, Kooraki S, Mohazab RA, et al. Epidemiological and clinical features of Brucella arthritis in 24 children. Ann Saudi Med 2011; 31: 270-273. https://doi.org/10.4103/0256-4947.81543
- Bosilkovski M, Kirova-Urosevic V, Cekovska Z, et al. Osteoarticular involvement in childhood brucellosis: experience with 133 cases in an endemic region. Pediatr Infect Dis J 2013; 32: 815-819. https://doi.org/10.1097/INF.0b013e31828e9d15
- al-Eissa YA, Kambal AM, Alrabeeah AA, Abdullah AM, al-Jurayyan NA, al-Jishi NM. Osteoarticular brucellosis in children. Ann Rheum Dis 1990; 49: 896-900. https://doi.org/10.1136/ard.49.11.896
- Gómez-Reino FJ, Mateo I, Fuertes A, Gómez-Reino JJ. Brucellar arthritis in children and its successful treatment with trimethoprim-sulphamethoxazole (co-trimoxazole). Ann Rheum Dis 1986; 45: 256-258. https://doi.org/10.1136/ard.45.3.256
- Çiftdoğan DY, Aslan S. Osteoarticular involvement of brucellosis in pediatric patients: clinical and laboratory characteristics. Turk J Pediatr 2020; 62: 199-207. https://doi.org/10.24953/turkjped.2020.02.005
- Unuvar GK, Kilic AU, Doganay M. Current therapeutic strategy in osteoarticular brucellosis. North Clin Istanb 2019; 6: 415-420. https://doi.org/10.14744/nci.2019.05658
- El Miedany YM, El Gaafary M, Baddour M, Ahmed I. Human brucellosis: do we need to revise our therapeutic policy? J Rheumatol 2003; 30: 2666-2672.
- Kayaaslan B, Bastug A, Aydin E, et al. A long-term survey of brucellosis: is there any marker to predict the complicated cases? Infect Dis (Lond) 2016; 48: 215-221. https://doi.org/10.3109/23744235.2015.1107187
- Shi QN, Qin HJ, Lu QS, et al. Incidence and warning signs for complications of human brucellosis: a multi-center observational study from China. Infect Dis Poverty 2024; 13: 18. https://doi.org/10.1186/s40249-024-01186-4
- Wang W, Wang Z, Jia K, Tang J, Wang L. Clinical and laboratory characteristics of childhood brucellosis in high-risk area of Western China. Jpn J Infect Dis 2022; 75: 127-132. https://doi.org/10.7883/yoken.JJID.2021.388
- Bray C, Bell LN, Liang H, et al. Erythrocyte sedimentation rate and c-reactive protein measurements and their relevance in clinical medicine. WMJ 2016; 115: 317-321.
- Crosby E, Llosa L, Miro Quesada M, Carrillo C, Gotuzzo E. Hematologic changes in brucellosis. J Infect Dis 1984; 150: 419-424. https://doi.org/10.1093/infdis/150.3.419
- Aydin E, Karadag MA, Cecen K, et al. Association of mean platelet volume and the monocyte/lymphocyte ratio with brucella-caused epididymo-orchitis. Southeast Asian J Trop Med Public Health 2016; 47: 450-456.
- Sen V, Bozkurt IH, Aydogdu O, et al. Significance of preoperative neutrophil-lymphocyte count ratio on predicting postoperative sepsis after percutaneous nephrolithotomy. Kaohsiung J Med Sci 2016; 32: 507-513. https://doi.org/10.1016/j.kjms.2016.08.008
- Peng J, Li H, Ou Q, et al. Preoperative lymphocyte-to-monocyte ratio represents a superior predictor compared with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for colorectal liver-only metastases survival. Onco Targets Ther 2017; 10: 3789-3799. https://doi.org/10.2147/OTT.S140872
- Olt S, Ergenç H, Açıkgöz SB. Predictive contribution of neutrophil/lymphocyte ratio in diagnosis of brucellosis. Biomed Res Int 2015; 2015: 210502. https://doi.org/10.1155/2015/210502
- Aktar F, Tekin R, Bektaş MS, et al. Diagnostic role of inflammatory markers in pediatric Brucella arthritis. Ital J Pediatr 2016; 42: 3. https://doi.org/10.1186/s13052-016-0211-5
- Rizo-Téllez SA, Méndez-García LA, Flores-Rebollo C, et al. The neutrophil-to-monocyte ratio and lymphocyte-to-neutrophil ratio at admission predict ın-hospital mortality in Mexican patients with severe SARS-CoV-2 infection (Covid-19). Microorganisms 2020; 8: 1560. https://doi.org/10.3390/microorganisms8101560
- Obaid JMAS, Almjydy MMA, Garban MAQ, Al-Hebari FSQ, Al-Washah NAH. Neutrophil-to-monocyte ratio is the better new inflammatory marker associated with rheumatoid arthritis activity. Health Sci Rep 2023; 6: e1478. https://doi.org/10.1002/hsr2.1478
- Xia X, Wang Y, Xie M, Qiu S, Zhou J. Elevated neutrophil - to - monocyte ratio as a prognostic marker for poor outcomes in neonatal sepsis. Heliyon 2022; 8: e11181. https://doi.org/10.1016/j.heliyon.2022.e11181
- Bozdemir ŞE, Altıntop YA, Uytun S, Aslaner H, Torun YA. Diagnostic role of mean platelet volume and neutrophil to lymphocyte ratio in childhood brucellosis. Korean J Intern Med 2017; 32: 1075-1081. https://doi.org/10.3904/kjim.2016.092
- Sen P, Demirdal T, Nemli SA. Predictive value of inflammation markers in Brucellosis. Arch Iran Med 2019; 22: 640-645.
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















