Abstract
Background. Vascular changes are observed in children with cystic fibrosis (cwCF), and gender-specific differences may impact arterial stiffness. We aimed to compare arterial stiffness and clinical parameters based on gender in cwCF and to determine the factors affecting arterial stiffness in cwCF.
Methods. Fifty-eight cwCF were included. Pulmonary function, lean body mass, handgrip strength, and peak oxygen uptake (VO2peak) were assessed using a cardiopulmonary exercise test. Arterial stiffness (pulse wave velocity [PWV] and augmentation index [AIx@75]) and hemodynamic parameters (resting heart rate [HR] and stroke volume [SV]) were measured using brachial pulse waves. Endothelial function (ICAM-1, sVCAM-1, sE-selectin, VEGF-A, ET-1) was evaluated using blood samples.
Results. Female cwCF had significantly lower VO2peak, SV, and PWV, and higher resting HR, AIx@75, and AIx@75-z-score than male cwCF (p<0.05). AIx@75-z-score was associated with gender (r=0.516, p<0.001), age (r= -0.345, p=0.008), lean body mass (r= -0.451, p<0.001), forced expiratory volume in one second (FEV1)-z-score (r= -0.332, p=0.011), handgrip strength (r= -0.466, p<0.001), and VO2peak (r= -0.459, p<0.001) and peak workload (r= -0.527, p<0.001). AIx@75-z-score was not associated with ICAM-1, sVCAM-1, sE-selectin, VEGF-A, or ET-1 (p>0.05). The FEV1-z-score and gender explained 34.6% of the variance in AIx@75-z-score (p<0.05).
Conclusions. Female cwCF have more impaired hemodynamics, less maximal exercise capacity, and increased arterial stiffness, indicating a higher cardiovascular risk compared to male cwCF. FEV1 and gender affect arterial stiffness in cwCF. Further studies are necessary to uncover the underlying factors for arterial stiffness and endothelial dysfunction and their clinical effects in cwCF.
Keywords: cystic fibrosis, maximal exercise capacity, endothelial dysfunction, arterial stiffness, pulse wave velocity
Introduction
Cystic fibrosis (CF) is a rare genetic disease resulting from mutations in the CF transmembrane conductance regulator (CFTR) gene.1 The CFTR is detected in endothelial cells derived from multiple organ systems, including the lung microvasculature.2 Impaired function of the CFTR in children with CF (cwCF) is associated with elevated cytokines and other inflammatory markers.3 CFTR activity plays a key role in maintaining vascular homeostasis, especially during an inflammatory response by the vascular endothelium.4 Increased endothelial permeability was observed in CF patients compared to healthy controls.5 Increased oxidative stress, inflammation, endothelial dysfunction, and life expectancy may increase cardiovascular risk.4
Arterial stiffness and endothelial dysfunction highlight different dimensions of vascular disease.6 The influence of systemic inflammation on endothelial function may contribute to the development of arterial stiffness.7 Airway inflammation has been implicated in causing endothelial dysfunction in the pulmonary circulation, which could contribute to systemic endothelial dysfunction.7 A reduction in pulmonary function may disrupt endothelial barrier function, which is directly affected by vascular wall stiffening.8 Few studies reported increased arterial stiffness9-12 and endothelial dysfunction13,14 in cwCF compared to healthy children.10 cwCF have enhanced aortic stiffness and wall thickness compared to controls.12 Increased arterial stiffness and endothelial dysfunction are associated with pulmonary function.9,13 These findings indicate that vascular changes observed in cwCF begin in early childhood. The arterial stiffness and endothelial function in cwCF have become even more critical, considering recent improvements in survival rates.
Arterial stiffness may be influenced by intrinsic gender differences.15 To date, no studies have directly compared arterial stiffness and endothelial function between female and male cwCF, despite evidence showing increased arterial stiffness in cwCF compared to healthy peers.9 The relationship between arterial stiffness and endothelial function, pulmonary function, and exercise capacity remains unclear. Therefore, we aimed (a) to compare the arterial stiffness, endothelial function, and clinical parameters, including physical characteristics, pulmonary function, peripheral muscle strength, and exercise capacity, between female and male cwCF and (b) to identify the factors affecting arterial stiffness in cwCF.
Material and Methods
Study design and population
All assessments were completed within a single day, with data collection taking place in the morning from 9 a.m. to 12 p.m. Ethical approval was obtained from the Hacettepe University, Non-Interventional Clinical Research Ethics Committee (Approval date: 07.01.2020, approval number: GO 19/1156). All participants and their parents signed informed consent forms. The study was registered on ClinicalTrials.gov (NCT04259983) and conducted in accordance with the Declaration of Helsinki.
Participants and procedures
This cross-sectional study was conducted between January 2020 and December 2023 at the Cardiopulmonary Rehabilitation Unit of the Hacettepe University, Faculty of Physical Therapy and Rehabilitation, in collaboration with the Hacettepe University, Faculty of Medicine (Department of Pediatric Pulmonology and Department of Physiology) and Faculty of Pharmacy (Department of Pharmaceutical Toxicology). Sixty-eight cwCF, aged 10–18 years, who were diagnosed and followed at the Department of Pediatric Pulmonology, Hacettepe University Faculty of Medicine, and referred to the Cardiopulmonary Rehabilitation Unit, were screened. The inclusion criteria were being 10–18 years old, clinically stable, able to cooperate with assessments, with forced expiratory volume in one second (FEV1) >40% predicted, not having experienced any exacerbations at least for three months, using regular medication for at least 12 months, and having no medication changes for at least three weeks. Exclusion criteria were having a resting oxygen saturation (SpO2) <92%, a history of smoking, having pulmonary surgery, having use of vasoactive drugs or oral steroids, having CF-related diabetes, having advanced orthopedic, neurologic, and cardiovascular diseases, and having a lower extremity injury (e.g., strain, sprain, or fracture) in the past six months.
Assessments
Age, gender, mutations, and medications were recorded. Lean body mass was evaluated using a skinfold caliper (Baseline Medical Skinfold Caliper, Fabrication Enterprises, NY, USA). Three measurements were taken from the biceps, triceps, subscapular, and supra iliac regions, and the mean values of the right side were used for analysis.16
Forced vital capacity (FVC), FEV1, peak expiratory flow (PEF), and forced expiratory flow from 25%–75% (FEF25–75%) were recorded from the medical records.17 Handgrip strength was measured using a portable dynamometer (Jamar, Nottinghamshire, UK). The right and left sides were measured thrice, and the best value was recorded.18
A cardiopulmonary exercise test (CPET) using Godfrey protocol19 was performed on an electronically braked bicycle ergometer (Lode, Corival CPET, Groningen, The Netherlands).13 The test was terminated in the instances of voluntary exhaustion, inability to maintain a 60-rpm cadence, or reaching the peak heart rate (HRpeak) and respiratory exchange ratio (RER) >1.03. The RER and peak oxygen consumption (VO2peak) were determined using gas exchange analysis (Quark CPET, COSMED, Rome, Italy) and HRpeak and peak workload (Wpeak) were recorded.
Evaluation of arterial stiffness
A portable device was used to evaluate arterial stiffness using brachial pulse waves (Tel-O-Graph BT, IEM GmbH, Aachen, Germany).20 The Tel-O-Graph, which uses an oscillometric principle, was employed to measure arterial stiffness. It enables blood pressure measurement with automatic transmission. A Bluetooth connection was established between the device and the data analysis software (Hypertension Management Software Client Server, HMS CS, Achen, Germany). Three consecutive measurements were taken for each child with CF, and the highest reading was used for evaluation. Pulse wave velocity (PWV), augmentation index normalized to heart rate with 75 beats/min (AIx@75), resting heart rate (HR), and stroke volume (SV) were evaluated.21 Augmentation index is an integrated measure that reflects both arterial wave reflection and systemic arterial stiffness.22 The AIx@75 was determined by assessing the aortic pressure wave and calculating the augmentation pressure, which is the difference between the peak of the reflected wave (P2) and the peak of the incident wave (P1). This value is expressed as a percentage of the central pulse pressure (cPP), calculated using the formula: AIx@75=(P2−P1)/cPP×100. We measured AIx@75 to minimize the influence of mean arterial pressure, age, gender, and HR on the augmentation index.9 Arterial stiffness measurements were performed after 12 hours of overnight fasting and before inhaler therapy in a sitting position after the patient had rested for 15 minutes in a quiet room.9
Evaluation of endothelial function
Blood samples were collected to assess endothelial function by measuring the levels of intercellular adhesion molecule-1 (ICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble endothelium-selectin (sE-selectin), vascular endothelial cell growth factor-A (VEGF-A), and endothelin-1 (ET-1). Samples were drawn via forearm venous puncture, collected into heparinized vacutainer tubes, and kept in sterile containers. Plasma samples were obtained through 10 minutes of centrifugation at 2000 rpm. All plasma samples were aliquoted into 2 mL Eppendorf tubes and stored at −80 °C until analyzed.23 Measurements of ICAM-1, sVCAM-1, sE-selectin, VEGF-A, and ET-1 levels were detected in plasma samples using an ELISA kit (Bioassay Technology Laboratory, Shanghai, China) following the manufacturer’s instructions with slight modifications. The absorbance of samples was read at λ=450 nm against a standard curve using a SpectraMax® M5 Microplate Reader (Molecular Devices LLC, San Jose, CA, USA). All experiments with indicators of endothelial dysfunction parameters were conducted with technical duplicates.
Statistical analyses
SPSS version 27.0 (IBM Corp. IBM SPSS Statistics for Windows, Armonk, NY, USA) was used for statistical analysis. Normality was checked using the Shapiro–Wilk test, and descriptive statistics were calculated. The analysis was performed using measured values, except for population-based pulmonary function test z-scores. Z-scores for weight,24 height24, heart rate25, systolic and diastolic blood pressure26, PWV26, and AIx@7525 were calculated. Data were presented as the median, interquartile range, mean and standard deviations, frequencies, and percentages, as appropriate. Student’s t-test or Mann-Whitney U test was used for the comparison, considering normality. Associations between AIx@75-z-score and the variables were analyzed using Pearson’s correlation coefficients. The statistical significance was set at p<0.05. A multiple linear regression analysis was performed. The scatter plots were created using GraphPad Prism v.8.0.2 (GraphPad Software, San Diego, CA, USA) and used to investigate the associations between the AIx@75-z-score and gender, age, lean body mass, FEV1-z-score, handgrip strength, VO2peak, and Wpeak. The variables showing a univariate association with the AIx@75-z-score (p<0.05) were initially entered into the multiple regression analysis (gender, handgrip strength, lean body mass, VO2peak, FEV1-z-score, and age).27 Since there was no statistical significance found regarding handgrip strength, VO2peak, and age with the model (p>0.05), the final model was established using gender and FEV1-z-score. For the final model, assumptions for variables were tested (normal distribution, heteroscedasticity, multicollinearity). The assumption of homoscedasticity, referring to the constancy of the residuals’ variance, was evaluated using a scatter plot of the residuals against the predicted values. The outcome dependent variable (AIx@75-z-score) was normally distributed, and the independent variables (FEV1-z-score and gender) did not violate assumptions. The post-hoc power was calculated using the G*Power program 3.1.9.7 (Franz Faul, Kiel University, Kiel, Germany) based on the comparison of AIx@75-z-score between female and male cwCF. The effect size and post-hoc power were found to be 1.19 and 99.38%, respectively.
Results
Sixty-eight cwCF were screened. Ten cwCF were excluded for the following reasons: missing data (n=2), declining to participate (n=6), and being identified as outliers (n=2). Therefore, 58 cwCF were included in the final analysis. Our study included 27 female and 31 male cwCF. Physical characteristics, CFTR mutations, lung treatments, pulmonary function, peripheral muscle strength, and cardiopulmonary exercise testing findings in cwCF are presented in Table I. The maximal exercise test was terminated in 56 cwCF due to a RER >1.03 (n=56) and in two cwCF due to an inability to maintain a 60-rpm cadence. None of the study participants were receiving modulator therapy or blood pressure medications.
|
Data are presented as median (interquartile range) or mean±standard deviation considering normality. *p<0.05. uMann-Whitney U test, tStudent’s t test, PPearson Chi-Square test, fFishers’s exact test. BMI: body mass index, cwCF: children with cystic fibrosis, FEF25 75%: forced expiratory flow from 25 to 75%, FEV1: forced expiratory volume in one second, FVC: forced vital capacity, HRpeak: peak heart rate, PEF: peak expiratory flow, VO2peak: peak oxygen uptake, Wpeak: peak workload. |
||||
| Table I. Physical characteristics, mutations, lung treatments, pulmonary function, peripheral muscle strength, and cardiopulmonary exercise testing in children with cystic fibrosis. | ||||
| Variables |
|
|
|
|
| Age (years) |
|
|
|
|
| CF diagnosis (months) |
|
|
|
|
| Weight (kg) |
|
|
|
|
| Weight-z-score |
|
|
|
|
| Height (cm) |
|
|
|
|
| Height-z-score |
|
|
|
|
| BMI (kg/m2) |
|
|
|
|
| BMI-z-score |
|
|
|
|
| Lean body mass (kg) |
|
|
|
|
| Mutations |
|
|||
| F508del homozygous, n (%) |
|
|
|
|
| F508del heterozygous, n (%) |
|
|
|
|
| Other mutations, n (%) |
|
|
|
|
| Lung treatments | ||||
| Pharmacological treatments | ||||
| Inhaled antibiotics, n (%) |
|
|
|
|
| Dornase alpha, n (%) |
|
|
|
|
| Inhaled corticosteroids, n (%) |
|
|
|
|
| Bronchodilators, n (%) |
|
|
|
|
| Hypertonic saline, n (%) |
|
|
|
|
| Airway clearance techniques |
|
|
|
|
| Pulmonary function testing | ||||
| FVC (L) |
|
|
|
|
| FVC-z-score |
|
|
|
|
| FEV1 (L) |
|
|
|
|
| FEV1-z-score |
|
|
|
|
| PEF (L) |
|
|
|
|
| PEF-z-score |
|
|
|
|
| FEF25-75% (L) |
|
|
|
|
| FEF25-75%-z-score |
|
|
|
|
| Peripheral muscle strength | ||||
| Handgrip strength (N) |
|
|
|
|
| Cardiopulmonary exercise testing | ||||
| VO2peak (mL/min) |
|
|
|
|
| Wpeak (Watt) |
|
|
|
|
| RER |
|
|
|
|
| HRpeak (bpm) |
|
|
|
|
| HRpeak %predicted (%) |
|
|
|
|
The age, weight-z-score, height-z-score, body mass index, lean body mass, mutations, lung treatments, FEV1, and FEV1%predicted, PEF, FEF25-75%, handgrip strength, RER, HRpeak, and HRpeak %predicted values were similar between female and male cwCF (p>0.05). Female cwCF had significantly lower FVC (mean±SD=2.73±0.74 L in females vs. 3.28±0.99 L in males; p=0.022), VO2peak (mean±SD= 1000.52±289.23 mL/min in females vs. 1390.94±388.53 mL/min in males; p<0.001), and Wpeak (median [interquartile range]=80.00 [60.00-100.00] Watt in females vs. 105.00 [90.00-140.00] Watt in males, p=0.002) compared to male cwCF. A comparison of arterial stiffness, hemodynamics, and endothelial function in female and male cwCF is presented in Table II. Female cwCF had significantly higher HR, AIx@75, and AIx@75-z-score with lower SV and PWV compared to those of male cwCF (p<0.05, Table II). ICAM-1, sVCAM-1, sE-selectin, VEGF-A, and ET-1 levels were similar between female and male cwCF (p>0.05, Table II).
|
Data are presented as median (interquartile range) or mean±standard deviation considering normality. *p<0.05. tStudent’s t-test. uMann-Whitney U test. AIx@75: augmentation index normalized to heart rate of 75 bpm, cwCF: children with cystic fibrosis, DBP: diastolic blood pressure, ET-1: endothelin 1, ICAM -1: intercellular adhesion molecule 1, PWV: pulse wave velocity, SBP: systolic blood pressure, sE-selectin: soluble endothelium-selectin, SV: Stroke volume, sVCAM-1: soluble vascular cell adhesion molecule 1, VEGF-A: vascular endothelial cell growth factor A. |
||||
| Table II. A comparison of arterial stiffness and endothelial function according to gender in children with cystic fibrosis. | ||||
| Variables |
|
|
|
|
| Hemodynamics | ||||
| Heart rate (bpm) |
|
|
|
|
| Heart rate-z-score |
|
|
|
|
| SBP (mmHg) |
|
|
|
|
| SBP-z-score |
|
|
|
|
| DBP (mmHg) |
|
|
|
|
| DBP-z-score |
|
|
|
|
| SV (mL) |
|
|
|
|
| Arterial stiffness | ||||
| PWV (m/s) |
|
|
|
|
| PWV-z-score |
|
|
|
|
| AIx@75 (%) |
|
|
|
|
| AIx@75-z-score |
|
|
|
|
| Endothelial function | ||||
| ICAM-1 (ng/L) |
|
|
|
|
| sVCAM-1 (ng/mL) |
|
|
|
|
| sE-selectin (ng/mL) |
|
|
|
|
| VEGF-A (ng/L) |
|
|
|
|
| ET-1 (ng/L) |
|
|
|
|
AIx@75-z-score showed a moderate correlation with gender (r=0.516, p<0.001), weak correlation with age (r= −0.345, p=0.008) and FEV1-z-score (r= −0.332, p=0.011), and moderate correlation with lean body mass (r= −0.451, p<0.001), handgrip strength (r= −0.466, p<0.001), VO2peak (r= −0.459, p<0.001), and Wpeak (r= −0.527, p<0.001). AIx@75-z-score was not associated with ICAM-1, sVCAM-1, sE-selectin, VEGF-A, and ET-1 (p>0.05).
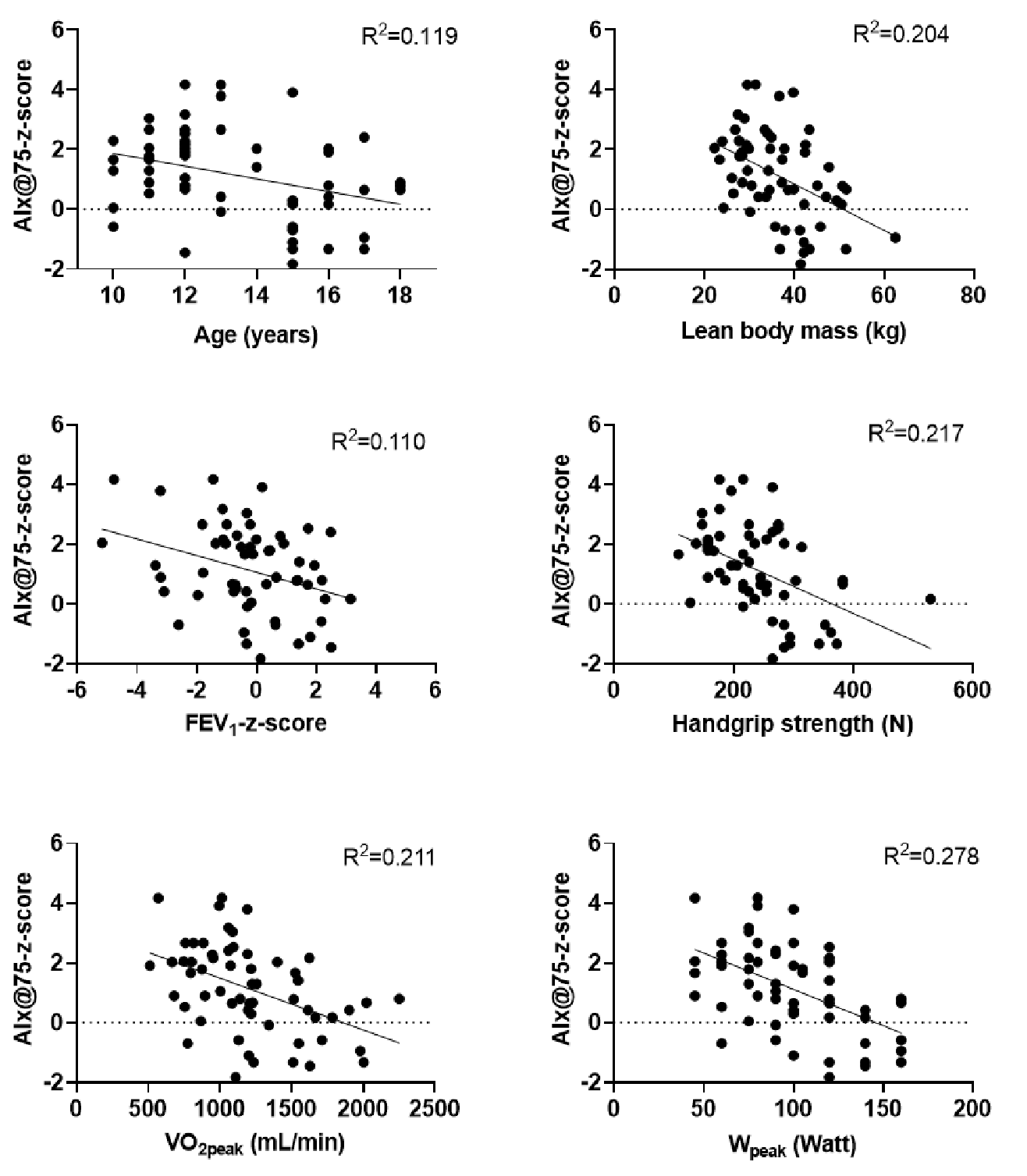
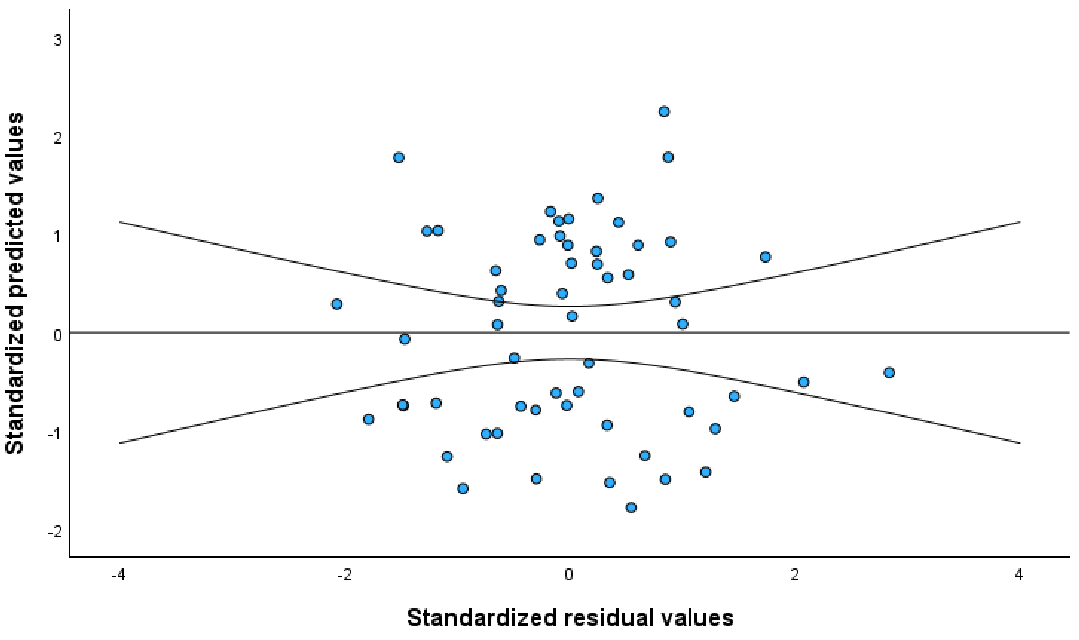
The scatter plots showing associations between AIx@75-z-score and age, lean body mass, FEV1-z-score, handgrip strength, VO2peak, and Wpeak are shown in Fig. 1. AIx@75-z-score was negatively associated with age (explaining 11.9% of the variance in AIx@75-z-score), lean body mass (explaining 20.4% of the variance in AIx@75-z-score), FEV1-z-score (explaining 11.0% of the variance in AIx@75-z-score), handgrip strength (explaining 21.7% of the variance in AIx@75-z-score), VO2peak (explaining 21.1% of the variance in AIx@75-z-score), and Wpeak (explaining 27.8% of the variance in AIx@75-z-score). A scatter plot of the predictive values of AIx@75-z-score against residuals is presented in Fig. 2. The residuals appeared to be randomly distributed without any specific pattern, indicating that there was no evidence of heteroscedasticity. The initial model is gender, handgrip strength, lean body mass, VO2peak, FEV1-z-score, and age (Table III). The final regression model with FEV1-z-score and gender explained 34.6% of the variance in AIx@75-z-score with statistical significance (Adjusted R2=0.346; Table IV, p<0.05) as shown in the following equation:
|
Initial model: F(6-51)=7.415, p<0.001, R2=0.466, Adjusted R2=0.403. AIx@75: augmentation index normalized to heart rate of 75 bpm, CI: confidence interval, FEV1: forced expiratory volume in one second, VIF: variance inflation factor, VO2peak: peak oxygen uptake. |
|||||
| Table III. Initial model with gender and handgrip strength, lean body mass, VO2peak, FEV1-z-score, and age as predictors of the AIx@75-z-score. | |||||
| Initial model |
|
|
|
|
|
| Constant |
|
|
|
||
| Gender (F/M) |
|
|
|
|
|
| Handgrip strength (N) |
|
|
|
|
|
| Lean body mass (kg) |
|
|
|
|
|
| VO2peak (mL/min) |
|
|
|
|
|
| FEV1-z-score |
|
|
|
|
|
| Age (years) |
|
|
|
|
|
|
*p<0.05. F(2-55)=16.082, R2=0.369, Adjusted R2=0.346. φModel summary with gender and FEV1-z-score as predictors. AIx@75: augmentation index normalized to heart rate of 75 bpm, CI: confidence interval, FEV1: forced expiratory volume in one second, VIF: variance inflation factor. |
|||||
| Table IV. Final model summary with gender and z-score of FEV1 as predictors of AIx@75. | |||||
| Final modelφ |
|
|
|
|
|
| Constant |
|
|
|
||
| Gender |
|
|
|
|
|
| FEV1-z-score |
|
|
|
|
|
AIx@75-z-score = 0.368 + 1.506 × Gender (females=1, males=0) − 0.269 × FEV1-z-score
When examining the individual relationships, the regression coefficients for gender and FEV1-z-score were statistically significant (p<0.05, Table IV). Females had 1.506 units higher AIx@75-z-scores than males. For a 1-unit increase in FEV1-z-score, AIx@75-z-score decreased by 0.269 units (Table IV).
Discussion
The present study reveals that airway obstruction (FEV1) and gender are the factors affecting arterial stiffness in cwCF. Female cwCF have higher arterial stiffness, impaired hemodynamics (resting HR and SV), and lower maximal exercise capacity, indicating a higher cardiovascular risk than male cwCF.
Regarding comparing hemodynamic parameters, arterial stiffness, and endothelial dysfunction between genders, female cwCF had a higher resting HR than males. The increase in resting HR may be a way to compensate for the decrease in SV. The SV decrease may result from the changes in the contractile properties of the heart since a reduction in both right and left ventricular function is reported in cwCF.10 The higher resting HR and lower SV in female cwCF might have led to lower VO2peak and Wpeak values in females compared to male cwCF.
Endothelial cell adhesion molecules of ICAM-1, VCAM-1, and sE-selectin play a role in the activation of inflammatory cells, their uptake and passage from vascular structures to the airways, and the development of airway inflammation.28 Angiogenesis is stimulated by tissue hypoxia, and VEGF-A is a potent angiogenic factor induced by inflammation and tissue hypoxia.29 Since the lungs clear ET-1, loss of functional pulmonary vascular channels and, thus, the decreased endothelial surface area may contribute to the decreased ability of the lung to remove ET-130 and the increased circulating ET-1 levels. Even though we did not observe any significant association between AIx@75 and endothelial markers as well as the differences between female and male cwCF in terms of endothelial markers, vascular endothelial dysfunction has been demonstrated in cwCF and adult CF relative to healthy peers.13,14 We believe that relatively younger age13, absence of cardiovascular disease risk factors such as CF-related diabetes and cardiovascular disease, colonization status, and relatively preserved airway function in most of the cwCF could be the main factors responsible for this finding.28 Further follow-up studies may clarify the clinical appearance and predictive value of endothelial dysfunction and its underlying factors with advancing age and disease severity in CF.
We observed that cwCF had relatively high AIx@75 values when compared with a study including healthy children with similar mean age (mean age=13.50±2.41, 37.30±9.15% in female and 25.94±11.15% in male cwCF vs. mean age=13.53±3.17 years, 22.60±8.00% in female and 21.80±7.97% in male healthy children).25 Furthermore, our findings revealed relatively low PWV values when compared with that of healthy individuals aged between 10-29 years (4.15±0.30 vs. 4.87±0.40 m/s [range: 4.25-5.25]).31 Despite low PWV, high AIx@75 in cwCF may result from the distinct mechanisms of each index, i.e., PWV reflects aortic stiffness, while AIx@75 reflects peripheral arterial tone.32 Factors such as inflammation and endothelial dysfunction may influence AIx@75 independently of PWV.32
Since PWV-z-score revealed no difference between female and male cwCF, we determined the individual contributors to AIx@75-z-score as AIx@75 is considered a more sensitive indicator of arterial aging in younger individuals than PWV.33 The regression analysis showed that FEV1-z-score and gender accounted for the change in AIx@75-z-score in cwCF. Regarding gender as a factor affecting AIx@75, compared with males of the same age, prepubescent females have stiffer large arteries, suggesting inherent genetic gender differences.15 When compared to males of the same age, prepubertal females were shown to have stiffer large arteries, which suggested natural genetic gender differences.15 The augmentation index decreases gradually with age in both genders, although the decrease significantly slows at the onset of puberty.34 Arterial stiffness is higher in females than in males during both the pubertal and post-pubertal periods as well as at 18 years of age.34 Differences in hormonal factors, metabolic and vascular responses may influence gender-based differences in cwCF.35 Gender differences in arterial stiffness are important for prognosis, as greater arterial stiffness has been shown to be associated with mortality, and this relationship is nearly twice as strong in females compared to males.36 In line with the literature, the higher AIx@75 and AIx@75-z-score in female cwCF compared with male cwCF in our study may indicate a higher risk in female cwCF for cardiovascular diseases. Being an independent factor, FEV1-z-score indicates that more severe airway disease is associated with greater arterial stiffness. FEV1 indicates the elastic fiber content of the lungs. In contrast, arterial stiffness reflects the aorta’s fragmentation of elastin and medial collagen content, essential structural proteins playing a role in the elastic recoil of the lungs and arteries.37 Since there is a balance between elastin and collagen production and their degradation, any variations in the volume and structure of these proteins result in dysfunction.37 A previous study showed a negative association between arterial stiffness and FEF25–75%.9 An association between pulmonary function and arterial stiffness at an early age might signify whether the basis for the association is developmental or genetic.
Maintaining a higher lean body mass can positively impact the cardiovascular health of young individuals.38 Handgrip strength, a surrogate measure of overall muscle strength, is considered a biomarker of aging.39 Low handgrip strength is associated with increased arterial stiffness across a wide age range, independent of gender and cardiovascular comorbidity.39 Lower aerobic capacity is related to higher resting HR and cardiovascular risk factors40 and increased arterial stiffness in children.41,42 Maturity and growth may influence the relationship between increased arterial stiffness and cardiorespiratory fitness at early ages.43 The associations between AIx@75-z-score and lean body mass, handgrip strength, and VO2peak support that promoting a high lean body mass, handgrip strength, and cardiorespiratory fitness in cwCF may have preventative roles against the development of arterial stiffness.
To the best of our knowledge, the present study is the first to comprehensively and timely evaluate vascular parameters in cwCF using a combined approach of oscillometric devices (reducing the human factor) and biochemical markers (different aspects of endothelial function) and investigate their relationship with clinical parameters, including exercise capacity and pulmonary function. Moreover, the present study enables a comparison of arterial stiffness and endothelial markers between female and male cwCF.
This study is subject to several limitations. First, the study’s cross-sectional nature prevented the observation of the clinical course of the endothelial function. A follow-up longitudinal study can highlight the clinical course of vascular function in cwCF. Second, our study included cwCF aged between 10-18 years with no cardiovascular diseases and mostly preserved lung function, i.e., only eight participants (13.8%) had an FEV1% predicted lower than 80%. While enabling a homogenous distribution in the study sample and mitigating the confounding effects, this situation may have masked potential associations, including the association between arterial stiffness and endothelial function. We did not evaluate the pubertal status of cwCF, and it was a limitation. Further studies with a wider sample size, incorporating pubertal status and adult participants considering cardiovascular comorbidities as well as follow-up studies, can provide a broader understanding of arterial stiffness and endothelial functions in CF.
In conclusion, female cwCF has higher resting HR, lower SV, lower VO2peak, and higher arterial stiffness, indicating a higher cardiovascular risk than males. Therefore, FEV1 and gender affect arterial stiffness in cwCF. Further follow-up studies with a larger sample size, including participants with cardiovascular comorbidities in CF, may help uncover the underlying factors for arterial stiffness and endothelial dysfunction and their effects in cwCF.
Ethical approval
The study was approved by Hacettepe University, Non-Interventional Clinical Research Ethics Committee (Approval date: 07.01.2020, approval number: GO 19/1156). This study was registered at ClinicalTrials.gov with identifier number NCT04259983. Informed consent forms signed by all participants and their parents.
Source of funding
This study was supported by the Scientific and Technological Research Council of Türkiye (TUBITAK) (Grant number 221s353).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet 2021; 397: 2195-2211. https://doi.org/10.1016/S0140-6736(20)32542-3
- Tousson A, Van Tine BA, Naren AP, Shaw GM, Schwiebert LM. Characterization of CFTR expression and chloride channel activity in human endothelia. Am J Physiol 1998; 275: C1555-C1564. https://doi.org/10.1152/ajpcell.1998.275.6.C1555
- VanDevanter DR, Kahle JS, O’Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros 2016; 15: 147-157. https://doi.org/10.1016/j.jcf.2015.09.008
- Totani L, Plebani R, Piccoli A, et al. Mechanisms of endothelial cell dysfunction in cystic fibrosis. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 3243-3253. https://doi.org/10.1016/j.bbadis.2017.08.011
- Lass JH, Spurney RV, Dutt RM, et al. A morphologic and fluorophotometric analysis of the corneal endothelium in type I diabetes mellitus and cystic fibrosis. Am J Ophthalmol 1985; 100: 783-788. https://doi.org/10.1016/s0002-9394(14)73367-7
- Anderson TJ. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol 2006; 22 Suppl B: 72B-80B. https://doi.org/10.1016/s0828-282x(06)70990-4
- Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002; 106: 653-658. https://doi.org/10.1161/01.cir.0000025404.78001.d8
- Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res 2015; 116: 895-908. https://doi.org/10.1161/CIRCRESAHA.116.305720
- Kartal Öztürk G, Conkar S, Eşki A, Gülen F, Keskinoğlu A, Demir E. Evaluation of increased arterial stiffness in pediatric patients with cystic fibrosis by augmentation index and pulse wave velocity analysis. Pediatr Pulmonol 2020; 55: 1147-1153. https://doi.org/10.1002/ppul.24688
- Eising JB, van der Ent CK, Teske AJ, Vanderschuren MM, Uiterwaal C, Meijboom FJ. Young patients with cystic fibrosis demonstrate subtle alterations of the cardiovascular system. J Cyst Fibros 2018; 17: 643-649. https://doi.org/10.1016/j.jcf.2017.12.009
- Buehler T, Steinmann M, Singer F, et al. Increased arterial stiffness in children with cystic fibrosis. Eur Respir J 2012; 39: 1536-1537. https://doi.org/10.1183/09031936.00212511
- Ververs FA, Eikendal ALM, Kofink D, et al. Preclinical aortic atherosclerosis in adolescents with chronic disease. J Am Heart Assoc 2022; 11: e024675. https://doi.org/10.1161/JAHA.122.024675
- Poore S, Berry B, Eidson D, McKie KT, Harris RA. Evidence of vascular endothelial dysfunction in young patients with cystic fibrosis. Chest 2013; 143: 939-945. https://doi.org/10.1378/chest.12-1934
- Kreslová M, Sýkorová A, Bittenglová R, et al. Age-related progression of microvascular dysfunction in cystic fibrosis: new detection ways and clinical outcomes. Physiol Res 2021; 70: 893-903. https://doi.org/10.33549/physiolres.934743
- Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab 2003; 88: 5375-5380. https://doi.org/10.1210/jc.2003-030722
- Andaki ACR, Quadros TMB, Gordia AP, Mota J, Tinôco ALA, Mendes EL. Skinfold reference curves and their use in predicting metabolic syndrome risk in children. J Pediatr (Rio J) 2017; 93: 490-496. https://doi.org/10.1016/j.jped.2016.11.013
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019; 200: e70-e88. https://doi.org/10.1164/rccm.201908-1590ST
- Beenakker EA, van der Hoeven JH, Fock JM, Maurits NM. Reference values of maximum isometric muscle force obtained in 270 children aged 4-16 years by hand-held dynamometry. Neuromuscul Disord 2001; 11: 441-446. https://doi.org/10.1016/s0960-8966(01)00193-6
- Kent L, O’Neill B, Davison G, et al. Cycle ergometer tests in children with cystic fibrosis: reliability and feasibility. Pediatr Pulmonol 2012; 47: 1226-1234. https://doi.org/10.1002/ppul.22578
- Reshetnik A, Gohlisch C, Tölle M, Zidek W, Van Der Giet M. Oscillometric assessment of arterial stiffness in everyday clinical practice. Hypertens Res 2017; 40: 140-145. https://doi.org/10.1038/hr.2016.115
- Parittotokkaporn S, de Castro D, Lowe A, Pylypchuk R. Carotid pulse wave analysis: future direction of hemodynamic and cardiovascular risk assessment. JMA J 2021; 4: 119-128. https://doi.org/10.31662/jmaj.2020-0108
- O’Rourke MF, Pauca AL. Augmentation of the aortic and central arterial pressure waveform. Blood Press Monit 2004; 9: 179-185. https://doi.org/10.1097/00126097-200408000-00002
- Kartal Y, Bozdemir Özel C, Çakmak A, et al. The relationship between lung function, exercise capacity, oxidant and antioxidant response in primary ciliary dyskinesia and cystic fibrosis. Turk J Pediatr 2024; 66: 309-322. https://doi.org/10.24953/turkjpediatr.2024.4581
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000; (314): 1-27.
- Santos LMD, Gomes IC, Pinho JF, et al. Predictors and reference equations for augmentation index, an arterial stiffness marker, in healthy children and adolescents. Clinics (Sao Paulo) 2021; 76: e2350. https://doi.org/10.6061/clinics/2021/e2350
- Elmenhorst J, Hulpke-Wette M, Barta C, Dalla Pozza R, Springer S, Oberhoffer R. Percentiles for central blood pressure and pulse wave velocity in children and adolescents recorded with an oscillometric device. Atherosclerosis 2015; 238: 9-16. https://doi.org/10.1016/j.atherosclerosis.2014.11.005
- Tabachnick BG, Fidell LS, Ullman JB. Chapter 5: Multiple regression. In: Using multivariate statistics. 7th ed. Boston: Pearson; 2013: 140.
- Declercq M, Treps L, Carmeliet P, Witters P. The role of endothelial cells in cystic fibrosis. J Cyst Fibros 2019; 18: 752-761. https://doi.org/10.1016/j.jcf.2019.07.005
- Watts KD, McColley SA. Elevated vascular endothelial growth factor is correlated with elevated erythropoietin in stable, young cystic fibrosis patients. Pediatr Pulmonol 2011; 46: 683-687. https://doi.org/10.1002/ppul.21428
- Siahanidou T, Nicolaidou P, Doudounakis S, Georgouli E, Papadimitriou A, Karpathios T. Plasma immunoreactive endothelin levels in children with cystic fibrosis. Acta Paediatr 2000; 89: 915-920. https://doi.org/10.1080/080352500750043332
- Kılıç A. Reference pulse wave velocity values in a healthy, normotensive Turkish population. Turk Kardiyol Dern Ars 2019; 47: 373-378. https://doi.org/10.5543/tkda.2019.92428
- Pessoa BP, Velloso M, Inácio ÉP, et al. Subclinical vascular, hemodynamic and arterial stiffness changes in adults with cystic fibrosis: cross-sectional observational study. Sci Rep 2024; 14: 13178. https://doi.org/10.1038/s41598-024-63904-0
- Lee HY, Oh BH. Aging and arterial stiffness. Circ J 2010; 74: 2257-2262. https://doi.org/10.1253/circj.cj-10-0910
- Hidvégi EV, Illyés M, Molnár FT, Cziráki A. Influence of body height on aortic systolic pressure augmentation and wave reflection in childhood. J Hum Hypertens 2015; 29: 495-501. https://doi.org/10.1038/jhh.2014.118
- Georeli E, Stamati A, Dimitriadou M, et al. Assessment of arterial stiffness in paediatric patients with type 1 diabetes mellitus. J Diabetes Complications 2024; 38: 108782. https://doi.org/10.1016/j.jdiacomp.2024.108782
- Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 2013; 61: 96-103. https://doi.org/10.1016/j.jacc.2012.08.997
- Zanoli L, Vancheri C. Lung dysfunction and increased arterial stiffness: causality or epiphenomenon? Angiology 2022; 73: 901-902. https://doi.org/10.1177/00033197221122838
- Muster V, Gütl K, Pregartner G, et al. Lower lean mass is associated with greater arterial stiffness in patients with lower extremity artery disease. J Pers Med 2021; 11: 911. https://doi.org/10.3390/jpm11090911
- König M, Buchmann N, Seeland U, Spira D, Steinhagen-Thiessen E, Demuth I. Low muscle strength and increased arterial stiffness go hand in hand. Sci Rep 2021; 11: 2906. https://doi.org/10.1038/s41598-021-81084-z
- Farah BQ, Christofaro DG, Balagopal PB, Cavalcante BR, de Barros MV, Ritti-Dias RM. Association between resting heart rate and cardiovascular risk factors in adolescents. Eur J Pediatr 2015; 174: 1621-1628. https://doi.org/10.1007/s00431-015-2580-y
- Haapala EA, Laukkanen JA, Takken T, Kujala UM, Finni T. Peak oxygen uptake, ventilatory threshold, and arterial stiffness in adolescents. Eur J Appl Physiol 2018; 118: 2367-2376. https://doi.org/10.1007/s00421-018-3963-3
- Veijalainen A, Tompuri T, Haapala EA, et al. Associations of cardiorespiratory fitness, physical activity, and adiposity with arterial stiffness in children. Scand J Med Sci Sports 2016; 26: 943-950. https://doi.org/10.1111/sms.12523
- Meyer J, Elmenhorst J, Giegerich T, Oberhoffer R, Müller J. Controversies in the association of cardiorespiratory fitness and arterial stiffness in children and adolescents. Hypertens Res 2017; 40: 675-678. https://doi.org/10.1038/hr.2017.19
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















