Abstract
Background. Food allergy is a public health concern affecting quality of life and increasing in prevalence. Numerous studies suggest that the rapid increase in the prevalence of allergic diseases may be linked to epigenetic mechanisms, particularly microRNA (miRNA), long non-coding RNA (lncRNA). The aim of this study was to investigate the effects of oxidative stress and selected non-coding RNAs on the development and pathogenesis of food allergy.
Methods. A total of 26 children with food allergy and 30 healthy children were enrolled in this study. Real-time polymerase chain reaction (RT-PCR) was performed to detect the expressions of serum miR-19a, miR-98 and lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in all the participants. Serum levels of interleukin-4 (IL-4), IL-10, IL-13 and transforming growth factor beta (TGF-β), along with levels of oxidative stress markers 8-isoprostane and cysteinyl leukotrienes, were measured by enzyme-linked immunosorbent assay.
Results. Our study found that the expression of miR-98 was significantly lower in children with food allergies compared to healthy controls (p < 0.05), whereas there was no significant difference in the expression levels of miR-19a between the two groups (p > 0.05). There was no difference in gene expression levels (p > 0.05) of lncRNA MALAT1 between children with food allergies and healthy children. TGF-β levels of healthy children were found to be significantly higher than those of children with food allergies (p < 0.05). There was no statistical difference in cysteinyl leukotriene levels between patients and controls (p = 0.804). However, 8-isoprostane levels were significantly lower in patients (6.68 pg/mL; interquartile range [IQR]: 1.57-26.55) compared to controls (37.20 pg/mL, IQR: 18.55-167.58) (p < 0.001).
Conclusions. Considering our findings in conjunction with existing literature, miR-98 appears to be a promising candidate biomarker for food allergy.
Keywords: epigenetics, food allergy, lncRNA MALAT1, miR-98, miR-19a, oxidative stress
Introduction
Food allergy is an inappropriate immune response that occurs after food intake or exposure.1 The prevalence of food allergies is increasing all over the world.2 The clinical findings of food allergies can range from mild itching to life-threatening anaphylaxis.3 Along with reducing the quality of life, food allergy consumes public health resources significantly.4,5 Depending on the type of immune response, food allergies are classified into three groups: immunoglobulin E-mediated (IgE-mediated), non-IgE-mediated food allergy, and mixed-type food allergy.6 IgE-mediated food allergies are the most common type and show immediate symptoms.7 During the sensitization phase of IgE-mediated food allergy, ingestion of the allergenic food protein triggers the production of food-specific IgE antibodies, which subsequently bind to tissue basophils and mast cells. When food crosses the disrupted barrier, dendritic cells are activated via danger signals and release inflammatory cytokines. These activated dendritic cells present the antigen to naïve T cells, the T cells differentiate into a T helper cell 2 (Th2) phenotype, which in turn promotes inflammatory signals that induce food antigen-specific B cells to class switch and produce food antigen-specific IgE. In the effector phase of IgE-mediated allergic reactions, re-exposure to the sensitized food allergen leads to mast cell degranulation and mediator release, triggered by cross-linking of the allergen with allergen-specific IgE bound to Fc epsilon receptor 1 (FcεRI) on mast cells. Therefore, mast cells release mediators such as histamine and leukotrienes and allergic reactions to food occur.8
Epigenetic changes are mechanisms that regulate genome activity that do not involve altering the DNA sequence.9 The main epigenetic mechanisms are divided into three as DNA methylation, histone modifications and non-coding RNAs.10 MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) are important regulators of gene expression, belonging to the class of non-coding RNAs.11
MiRNAs are untranslated transcripts found in clusters within the introns of other genes.12 miRNAs are about 18-26 nucleotides long and are responsible for the translational repression of messenger RNA (mRNA). They cause translational repression by partial binding to the non-protein-translated region (3’-UTR) located at the end of the target mRNA via RNA-induced silencing complex (RISC), or mRNA degradation by full conjugation.13 MiRNAs play a role in biological processes such as apoptosis, cancer, cell differentiation, and inflammation. MiRNAs are also involved in the regulation of the immune response. MiRNAs affect the development of diseases or the strength of inflammation in the affected tissues.14
LncRNAs are non-coding RNAs longer than 200 nucleotides. It is estimated that there are approximately 16,000 lncRNAs in the human genome.15 LncRNAs are structurally similar to mRNAs, but lncRNAs do not have an open reading frame (ORF). Therefore, they cannot code for proteins.16 LncRNAs are found in the nucleus and cytoplasm and are involved in the regulation of almost every step of gene expression by various mechanisms. As epigenetic modulators, lncRNAs bind to chromatin-modifying enzymes and direct their activity to specific regions of the genome. In these regions, lncRNAs direct chromatin modification by changing the pattern of gene expression. LncRNAs act on transcription factors and cause suppression or activation of the target gene. LncRNAs regulate alternative splicing by acting on splice factors. In addition, lncRNAs prevent the miRNA from binding to the target mRNA by acting as a sponge between the target mRNA and the miRNA.17 Emerging evidence suggests that lncRNAs are involved in the transcriptional or epigenetic regulation of gene expression, various biological processes such as cell differentiation, embryonic development, cancer metabolism, and inflammation.18
The expression pattern of miRNAs and lncRNAs may vary according to different cell types and disease conditions. miRNAs and lncRNAs are stable and detectable in different body fluids such as serum, urine and saliva. Furthermore, it has been shown that miRNAs and lncRNAs may play a role in the prognosis of the disease, in predicting the response to the treatment, and following the disease process. Therefore, it is thought that miRNAs and lncRNA can be used as biomarkers in airway and allergic diseases.14,19
In our study, we aimed to identify candidate biomarkers that could be used to distinguish children with food allergy from healthy controls and to determine the effects of selected non-coding RNAs on inflammation. Additionally, by identifying a potential diagnostic biomarker for food allergy, we sought to reduce the reliance on food challenge tests, which are both challenging and carry inherent risks. We also aimed to explore the relationship between food allergy and oxidative stress.
Materials and Methods
Patients
Twenty-six children with IgE-mediated food allergy from the Division of Allergy, and 30 healthy children from the Department of Child Health and Diseases General Outpatient Clinic who agreed to participate in the study were enrolled between June 2020 and July 2021, at İhsan Doğramacı Children’s Hospital of Hacettepe University, Ankara, Türkiye. All patients and parents gave their informed written consent to participate in the study. The criteria for inclusion in the food allergy group were as follows: food-specific IgE ≥0.35 IU/L, a swelling of 3 mm or more compared to the negative control in the epidermal prick test and the presence of clinical symptoms. In the healthy control group, the criteria for inclusion in the study were the absence of food allergy and atopy. Blood samples were collected from all study participants for miRNA and mRNA isolation from serum. The study was approved by Hacettepe University Non-interventional Clinical Research Ethics Committee (approval no. GO 20/343).
The demographic characteristics of the study population are given in Table I.
| SD: standard deviation | |||
| Table I. The demographic and clinical characteristics of the study population. | |||
|
|
|
|
|
| Age (year), mean±SD |
|
|
|
| Female sex, n (%) |
|
|
|
| Total IgE (IU/mL), median (Q1-Q3) |
|
|
|
| Eosinophil count (x103/µL), median (Q1-Q3) |
|
|
|
| Eosinophils (%), median (Q1-Q3) |
|
|
|
| Food allergy pattern, n (%) |
|
||
| Milk |
|
||
| Milk and egg white |
|
||
| Nuts |
|
||
| Milk and nuts |
|
||
MiRNA and lncRNA isolation and quantification
The serum samples were separated from the blood by centrifugation and stored at -80°C until use for miRNA isolation by miRNeasy Serum/Plasma Kit (QIAGEN, Germany).
MiRNAs were reversely transcripted to complementary DNA (cDNA) according to the instructions of the miScript II RT Kit (QIAGEN, Germany) by using HiFlex Buffer, which allows reverse transcription of both mRNA and miRNA into cDNA. The expression levels of miR-19a and miR-98 were derermined by using the miScript SYBR Green Kit (QIAGEN, Germany). The primer assays for miR-98, miR-19a, and miR-16 were purchased from QIAGEN. Maxima SYBR Green/ROX qPCR Master Mix” kit (ThermoFisher Scientific, USA) was used to determine expression levels of lncRNA MALAT1 and elongation factor 1-alpha (EF1-α), housekeeping gene. Primers for MALAT1 and EF1-α were purchased from Integrated DNA Technologies (Iowa, USA). The expression analyzes were performed by using Applied Biosystems Fast 7500 Real Time PCR System device. The relative expression levels of each miRNA were normalized by endogenous miR-16, and EF1-α was used as a housekeeping gene for lncRNA MALAT1. Differentiation in expression levels between samples was revealed by using the 2-∆∆Ct method.
Enzyme-linked immunosorbent assay (ELISA)
The serum levels of interleukin IL-4, IL-10, transforming growth factor beta (TGF-β) and IL-13, 8-isoprostane and cysteinyl leukotrienes were measured by ELISA by using commercial kits, according to the manufacturer’s instructions. Briefly, standards and samples were added to the wells of the capture antibody-coated plates and incubated at room temperature for the specified time. Unbound molecules were then washed away, and the enzyme-conjugated secondary antibody was added and incubated at room temperature for the specified time. After the washing process, the enzyme-specific substrate was added and incubated at room temperature for the specified time. After the reaction was stopped, measurements were made at the appropriate wavelengths. The concentrations of the samples were determined by using the standard graphs obtained.
Statistical analysis
The SPSS 22 for Windows program was used for statistical analysis. The comparison of numerical variables was performed using parametric or non-parametric tests depending on whether they were normally distributed. Categorical variables were evaluated using the chi-square test or Fisher’s exact test. Expressions of target miRNAs and lncRNA were normalized according to the expression of control miRNA and EF-1α, respectively. The results were analyzed using the 2−ΔΔCt method. The results are given as fold change. The Mann-Whitney U test was used to analyze expression levels. In all analyses, p < 0.05 was considered statistically significant.
Results
Expression levels of miRNAs
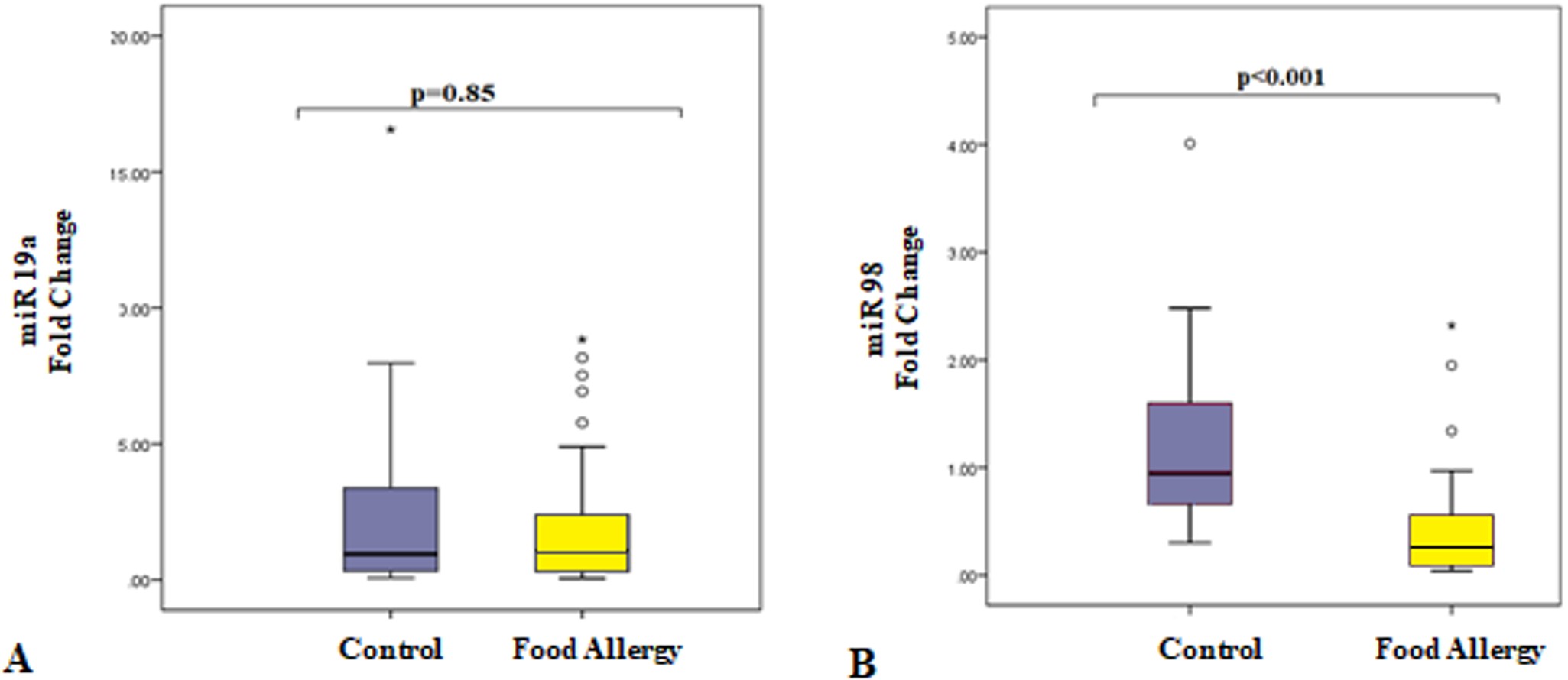
The expression levels of selected miRNAs miR-19a, and miR-98 were compared between children with food allergies and healthy controls. The mean of the ∆Ct values of the control group was calculated. The miRNA expression level of the individual whose ∆Ct value was closest to the mean ∆Ct values of the control group was accepted as 2-ΔΔCt = 1, and changes in expression levels of other individuals in the control group and food allergy patients were compared with the expression level of this reference individual.
There was no difference in the level of miR-19a expression between children with food allergies and healthy children (p=0.85, Fig. 1A), while the expression levels of miR-98 were significantly downregulated in children with food allergy compared with healthy children. (p<0.001, Fig. 1B).
Expression levels of lncRNA MALAT1
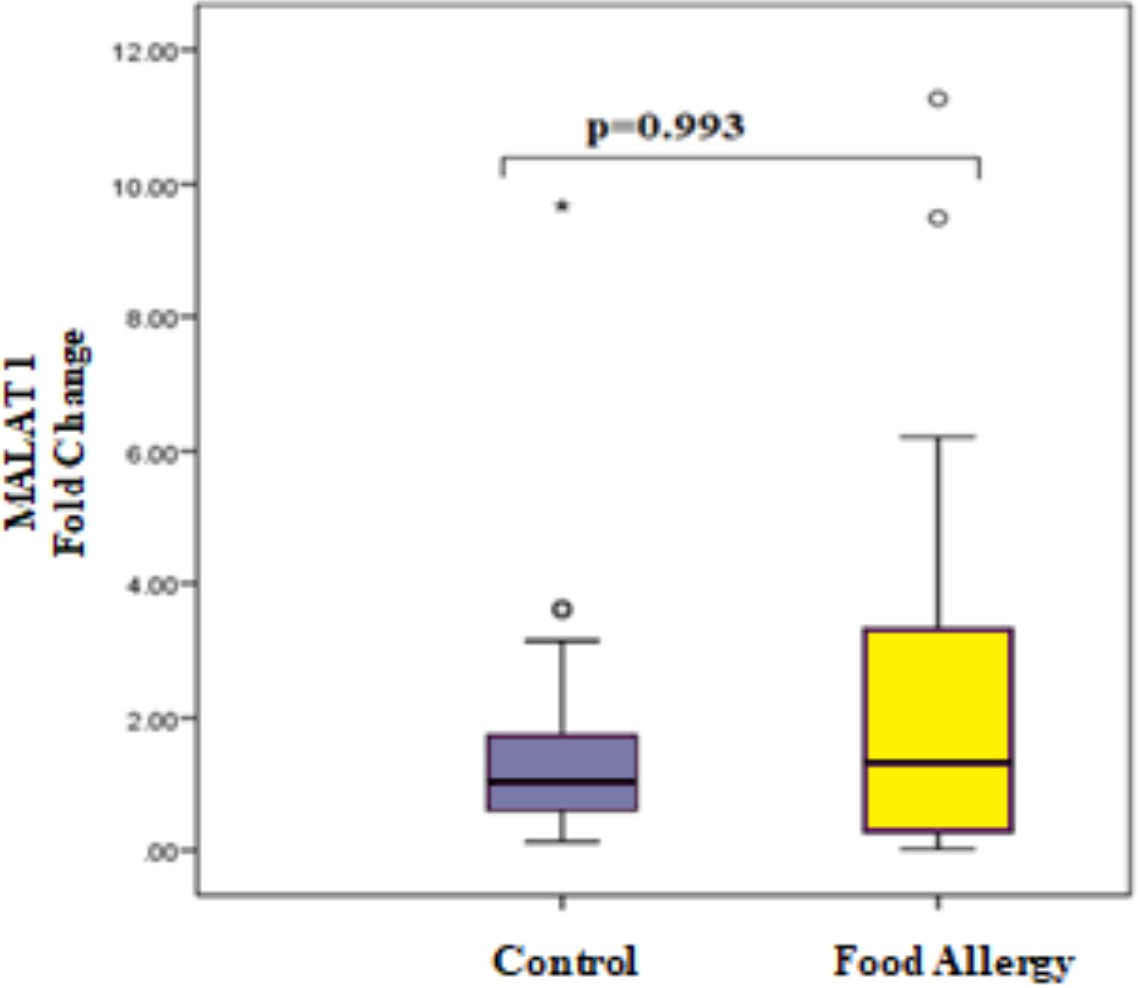
Although MALAT1 expression levels seem to be higher in patients, no significant difference was observed between the patient and control groups due to high inter-sample variability (p = 0.993, Fig. 2).
Cytokine levels
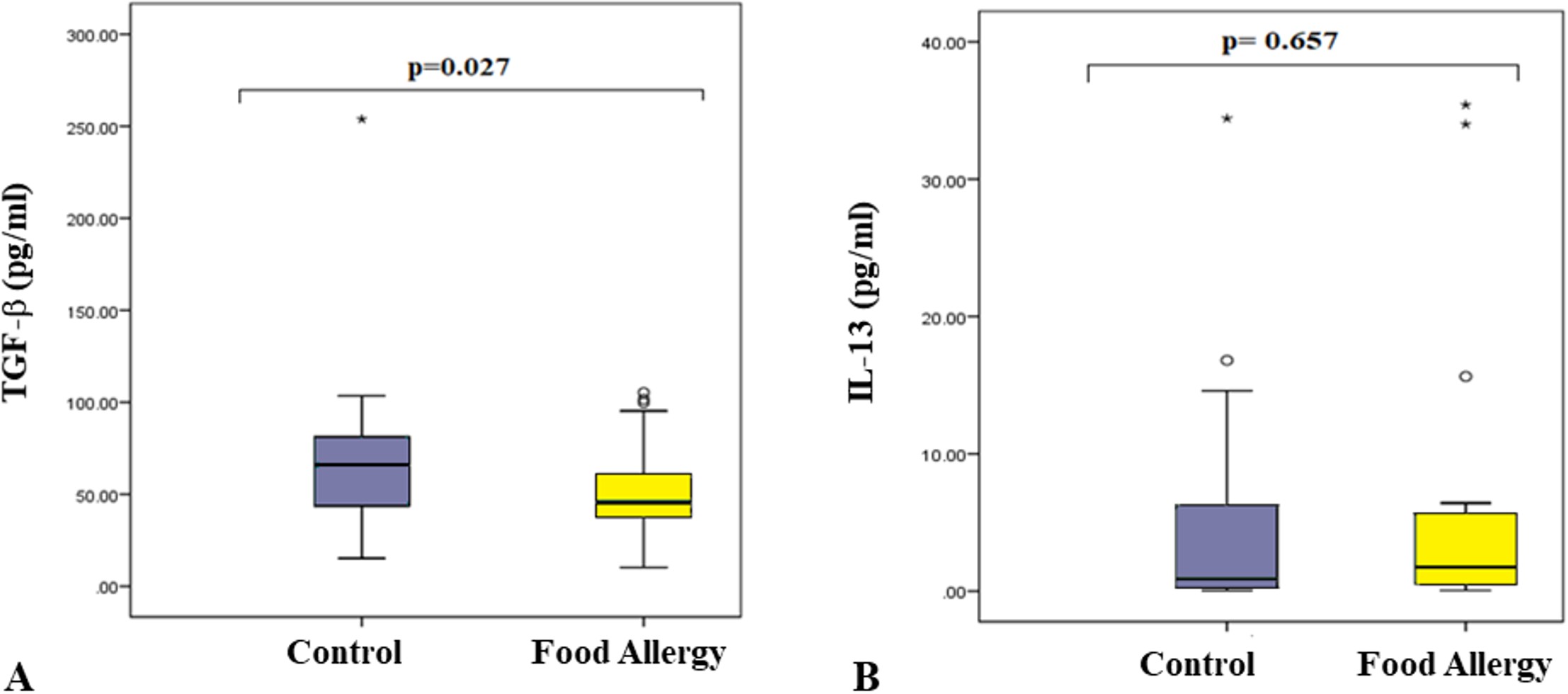
The serum level of TGF-β was found to be significantly lower in the patient group compared to the control group (p=0.027, Fig. 3A). In contrast, the IL-13 protein levels in the serum samples of the patient group were not different from the control group (p=0.85, Fig. 3B). IL-4 and IL-10 proteins were not detectable at measurable levels in the serum samples of either group.
Oxidative stress markers
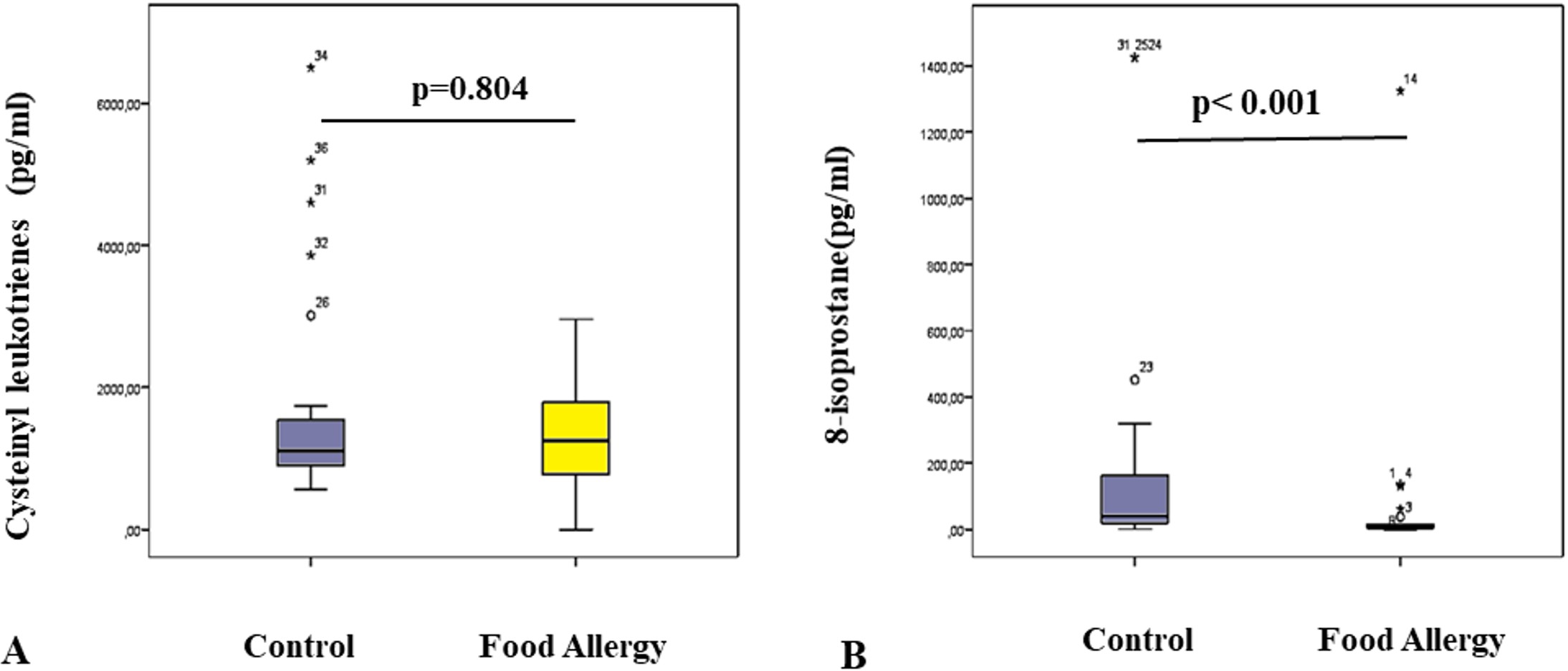
There was no statistical difference in cysteinyl leukotriene levels (p=0.804) between controls and patients (Fig. 4A). However, 8-isoprostane levels were significantly lower in patients (6.68 pg/mL; interquartile range [IQR]: 1.57-26.55) compared to controls (37.20 pg/mL; IQR: 18.55-167.58) (p< 0.001, Fig. 4B).
Discussion
Food allergy, defined as an inappropriate immune response to a harmless food antigen, is an important disease that can recur when exposed to the same food and can be life-threatening.20 The prevalence of food allergy and other allergic diseases is increasing all over the world.2 A growing body of evidence suggests that this increase may be associated with epigenetic mechanisms, particularly involving miRNA and lncRNA.
MiR-19a, a member of the miR-17~92 cluster, promotes Th2 cytokine production by simultaneously targeting inhibitors of the NF-KB, JAK-STAT and PI3K pathways. It has also been observed that mir-19a is increased in allergic inflammation and supports the production of IL-5 and IL-13.21 Another study revealed that the levels of thrombospondin-1 (TSP1), molecule involved in the maintenance of immune tolerance, were significantly decreased, while the levels of miR-19a were significantly increased in intestinal CD35+ B cells of mice sensitized to ovalbumin (OVA) as compared to naïve controls. They concluded that IL-4 suppresses the expression of TSP1 in the intestinal CD35+ B cells by up regulating miR-19a.22
In a study that aimed to investigate the role of miR-17-92 cluster in the induction of food allergen-related inflammation in the intestine, the authors found that the levels of miR-19a were significantly higher in the B cells of the intestine of food allergic mice than those in naïve control mice. They also showed that exposure of B cells, which were isolated from the mouse spleen, to IL-4 in the culture led to increased expression of miR-19a and suppressed expression of IL-10 in these cells.23
As a result of our study, when miR-19a gene expression levels were compared between the patient and control groups, there was no significant difference (p > 0.05). However, a negative correlation was found between miR-19a levels and patient eosinophil count (p=0.009, r= -0.505).
MiR-98, another miRNA selected in our study, is an important member of the Let-7 family. MiR-98 may serve as a regulator of the T cell mediated immune response.24 Xie and Xu25 found that patients with systemic lupus erythematosus had lower expression of miR-98 and higher Fas mRNA and protein levels in CD4+ T cells compared to healthy donors. In the study by Xie et al.26 CD4+ cells were isolated from the lamina propria mononuclear cells of mice exposed to peanut extract and the expression level of miR-98 was examined. As a result of the study, an increase in miR-98 expression level was found.
Luo et al.27 showed that the levels of IL-10 in peripheral B cells were significantly lower in patients with airway allergy as compared with healthy subjects. High levels of miR-98 were detected in peripheral B cells of patients when the B cells were stimulated with IL-4 to mimic allergy status. In this study, it was shown that miR-98 mediated IL-4 inhibited IL-10 expression in B cells.
In a study performed by Chen et al.28 the levels of miR-98 were found to be higher, but the levels of TSP1 were lower, in B cells isolated from the peripheral blood in patients with asthma. A negative correlation was identified between the levels of miR-98 and TSP1 in B cells.
In our study, when miR-98 gene expression levels were compared between children with food allergy and healthy controls, statistically significant differences were found between the two groups (p<0.05). The expression results of miR-98 we obtained are inconsistent with the results obtained in other studies in the literature. But we also found a positive correlation (p= 0.032, r= 0.494) between miR-98 levels and IL-13 levels in patients with food allergy. When we evaluated both groups together, no correlation was found between miR-98 levels and IL-13 levels (p= 0.273, r= 0.175).
MALAT1 is a prominent intergenic lncRNA known to be associated with metastasis in non-small cell lung cancer.29 Through its essential role in T helper cell differentiation and function, MALAT1 has important roles in immune response.30 MALAT1 regulates the innate immune response.31 In lung tissue biopsy samples taken from patients with chronic obstructive pulmonary disease (COPD) and a control group, MALAT1 expression levels of patients with COPD were found to be significantly higher than those of the control group.32 In the study by Qiu et al.33 it was observed that MALAT1 was expressed more in CD4+ cells from asthmatic patients compared to healthy patients. A recent study used bioinformatics to uncover the lncRNA-miRNA-mRNA regulatory network of bronchial epithelial cells in severe asthma. Five mRNA datasets from bronchial brushing samples from severe asthmatic patients and healthy controls were downloaded from the Gene Expression Omnibus (GEO) database, and MALAT1 was identified as one of the top 10 competing endogenous RNAs (ceRNAs) upon analysis.34
A study conducted by Yu et al. investigated Morinda officinalis extract (MOE) and its interaction with the long non-coding RNA MALAT1 in the treatment of atopic dermatitis. They showed that MOE inhibited MALAT1 expression in atopic dermatitis, leading to reduced expression of C-C chemokine receptor type 7 (CCR7), which is regulated through a ceRNA mechanism involving MALAT1 acting as a sponge for miR-590-5p. This, in turn, suppressed tumor necrosis factor alpha (TNF-α) / interferon gamma (IFN-γ)-induced cellular proliferation and inflammation.35 In another study, the authors demonstrated that MALAT1 increased NLRP3 expression by targeting miR-124-3p in a mouse model of atopic dermatitis. They reported that suppression of MALAT1 suppressed NLRP3 inflammasome activation and attenuated the Th1/Th2 imbalance in Th2-conditioned CD4+ T cells.36 Feng and colleagues showed that MALAT1 was highly expressed in mice with food allergy, and its silencing relieved allergic reactions with reduction in intestinal inflammatory cells and mast cells in food allergy mice by using BALB/c mice that were sensitized to ovalbumin in accordance with a model of food allergy protocol. They also found that MALAT1 aditionally promotes IL-6 secretion by dendritic cells and their maturation. As a result of their findings the authors suggested that therapeutically blocking MALAT1 in food allergy could reduce the severity of food allergy by decreasing the secretion of IL-6 by dendritic cells and suppressing the immunomodulation of T regulatory cells.37
In our study, there was no difference between the two groups in the expression levels of MALAT1 (p>0.05). When we examine the existing literature, most studies have utilized tissue samples or inflammatory cells. In contrast, our study used serum samples, which may explain why we did not observe any significant differences between the groups.
We determined that the 3′-UTRs of the IL-6, IL6R, IL-8, IL-10, IL-13, TGFBR1, TGFBR3 and IL22RA1 genes contain putative binding sites of miR-98 using target estimation programs TargetScan (http://www.targetscan.org/), miRDB (http://mirdb.org/), and miRBase (https://www.mirbase.org/). Among these target genes we selected IL-10, IL-13, TGF-β, which are effective in allergic diseases and the pathogenesis of food allergy, and IL-4, which has been shown to be associated with miR-98 in the literature, to investigate the role of miRNAs in disease pathogenesis. Protein levels were measured by the ELISA method. When the TGF-β protein levels were examined, there was a statistically significant difference between the patient and control groups (p<0.05).
TGF-β, the main regulator of the immune response, has important anti-inflammatory and immunosuppressive functions. TGF-β, which has a chemoattractant effect, leads to rapid accumulation of macrophages, granulocytes, and other cells at the site of inflammation. TGF-β stimulates the secretion of other inflammatory cytokines, and in the meantime recruits granulocytes to the site, which strengthens the immune response. TGF-β1 inhibits immune cell differentiation (Th1 and Th2 cells and B cells) and cytokine production (IFN-γ and IL-2) and is also involved in the development and differentiation of T regulatory cells.38 In a study that included 37 patients with allergic rhinitis and 30 healthy people, TGF-β protein levels were found to be significantly lower in patients with allergic rhinitis than in healthy controls.39 The concentration of TGF-β-1 was found to be significantly higher in patients with rhinosinusitis compared to the control group. In our study, TGF-β level was found to be significantly higher in the control group.40 This finding is in line with the data in the literature due to the anti-inflammatory properties of TGF-β and its effects on the differentiation and development of Treg cells.
In our study, IL-4 and IL-10 proteins were not found in the samples at a measurable level, however, no significant difference was observed between food allergy patients and the control group in terms of IL-13 protein levels (p>0.05).
Oxidative stress is often defined as an imbalance of pro-oxidants and antioxidants, which causes damage to cells or tissues. 8-isoprostane, a marker of oxidative stress, is a non-enzymatic peroxidation product of arachidonic acid. Levels of 8-isoprostane were found to be higher than normal in the exhaled breath condensate (EBC) of asthmatic adults or children.41 It has also been shown that elevated levels of 8-isoprostane is associated with exercise-induced bronchoconstriction in asthmatic children and adolescents.42 In our study, contrary to the literature, 8-isoprostane levels were found to be significantly lower in patients than in controls. The available data in the literature primarily stem from studies on other allergic conditions, such as asthma and atopic dermatitis, and to the best of our knowledge, no study has specifically investigated isoprostane levels in food allergy. Due to the small sample size in our study and the lack of comparable data in the literature, the relationship between oxidative stress and food allergy remains unclear. Further comprehensive studies are needed to elucidate this connection.
Our study has several limitations. The most significant is the small sample size in the study groups. Additionally, the lack of correlation analysis between miRNA expression levels and inflammatory markers, such as cytokines and oxidative stress indicators, represents a notable gap in our investigation. One of the aims of the study was to find a biomarker that could distinguish those with food allergies from those who are healthy, thereby reducing the need for risky and laborious food provocation tests. However, the data obtained at the end of the study, although weak, indicated that miR-98 alone could be a candidate for this purpose. Another weakness of our study is the lack of a comparison group for allergic diseases such as asthma, in addition to a healthy control group. Including another allergic disease group would have allowed us to determine whether miR-98, which was prominent in our study, is a food allergy-specific biomarker.
Another limitation of our study is that blood samples were collected outside the context of active food allergic reactions. This may partly explain the discrepancies between our findings and those reported in the literature. We believe that conducting future studies using samples collected both before and after a food challenge test with the suspected allergen would provide a clearer understanding of the relationship between food allergies and non-coding RNAs.
One of the strengths of our study is the limited number of clinical studies demonstrating the relationship between food allergies and non-coding RNAs. Most of these studies are related to milk and peanut allergies. The miRNAs we selected, miR19a and miR-98, have been previously shown to be associated with food allergy in animal models and have not previously been included in clinical studies. In this respect, despite the small sample size, they contribute new data to the literature. Similarly, as mentioned above, to the best of our knowledge, our study is the first to date to explore the relationship between food allergy and oxidative stress.
In conclusion, when considered alongside the existing literature, miR-98 may serve as a potential biomarker for food allergy. There is a need to replicate these findings in a larger patient population and to further investigate the relationship between miR-98 and inflammatory markers.
Ethical approval
The study was approved by Hacettepe University Non-interventional Clinical Research Ethics Committee (date: 17.04.2020, number: GO 20/343). Informed consent was obtained from all individual participants before included in the study.
Source of funding
This study was supported by Hacettepe University Scientific Research Projects Coordination Unit (project no: FYL-2020-18798).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol 2010; 125: S116-S125. https://doi.org/10.1016/j.jaci.2009.08.028
- Bartha I, Almulhem N, Santos AF. Feast for thought: a comprehensive review of food allergy 2021-2023. J Allergy Clin Immunol 2024; 153: 576-594. https://doi.org/10.1016/j.jaci.2023.11.918
- Hong JY, Li SS, Hu TY, et al. Frontline science: TLR3 activation inhibits food allergy in mice by inducing IFN-γ+ Foxp3+ regulatory T cells. J Leukoc Biol 2019; 106: 1201-1209. https://doi.org/10.1002/JLB.3HI0918-348RR
- Baiardini I, Braido F, Brandi S, Canonica GW. Allergic diseases and their impact on quality of life. Ann Allergy Asthma Immunol 2006; 97: 419-428. https://doi.org/10.1016/S1081-1206(10)60928-3
- Dyer AA, Negris OR, Gupta RS, Bilaver LA. Food allergy: how expensive are they? Curr Opin Allergy Clin Immunol 2020; 20: 188-193. https://doi.org/10.1097/ACI.0000000000000622
- Genel S, Floca E, Sur L. Food allergy: always a threat, how do we treat it? Pharmaceut Anal Acta 2013; 4: 268. https://doi.org/10.4172/2153-2435.1000268
- Cosme-Blanco W, Arroyo-Flores E, Ale H. Food allergies. Pediatr Rev 2020; 41: 403-415. https://doi.org/10.1542/pir.2019-0037
- Waserman S, Watson W. Food allergy. Allergy Asthma Clin Immunol 2011; 7(Suppl 1): S7. https://doi.org/10.1186/1710-1492-7-S1-S7
- Skinner MK, Nilsson EE. Role of environmentally induced epigenetic transgenerational inheritance in evolutionary biology: unified evolution theory. Environ Epigenet 2021; 7: dvab012. https://doi.org/10.1093/eep/dvab012
- Wei JW, Huang K, Yang C, Kang CS. Non-coding RNAs as regulators in epigenetics (Review). Oncol Rep 2017; 37: 3-9. https://doi.org/10.3892/or.2016.5236
- Kaikkonen MU, Lam MT, Glass CK. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc Res 2011; 90: 430-440. https://doi.org/10.1093/cvr/cvr097
- Bhat SS, Jarmolowski A, Szweykowska-Kulińska Z. MicroRNA biogenesis: epigenetic modifications as another layer of complexity in the microRNA expression regulation. Acta Biochim Pol 2016; 63: 717-723. https://doi.org/10.18388/abp.2016_1370
- O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018; 9: 402. https://doi.org/10.3389/fendo.2018.00402
- Akbas F, Coskunpinar E, Aynaci E, Oltulu YM, Yildiz P. Analysis of serum micro-RNAs as potential biomarker in chronic obstructive pulmonary disease. Exp Lung Res 2012; 38: 286-294. https://doi.org/10.3109/01902148.2012.689088
- He Q, Long J, Yin Y, et al. Emerging roles of lncRNAs in the formation and progression of colorectal cancer. Front Oncol 2020; 9: 1542. https://doi.org/10.3389/fonc.2019.01542
- Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 2012; 22: 1775-1789. https://doi.org/10.1101/gr.132159.111
- Malik B, Feng FY. Long noncoding RNAs in prostate cancer: overview and clinical implications. Asian J Androl 2016; 18: 568-574. https://doi.org/10.4103/1008-682X.177123
- Chen J, Ao L, Yang J. Long non-coding RNAs in diseases related to inflammation and immunity. Ann Transl Med 2019; 7: 494. https://doi.org/10.21037/atm.2019.08.37
- Ghafouri-Fard S, Shoorei H, Taheri M, Sanak M. Emerging role of non-coding RNAs in allergic disorders. Biomed Pharmacother 2020; 130: 110615. https://doi.org/10.1016/j.biopha.2020.110615
- Mahmoudi M. Allergy and Asthma: The Basics to Best Practices. Switzerland: Springer Nature; 2019. https://doi.org/10.1007/978-3-030-05147-1
- Simpson LJ, Patel S, Bhakta NR, et al. A microRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat Immunol 2014; 15: 1162-1170. https://doi.org/10.1038/ni.3026
- Yang LT, Li XX, Qiu SQ, et al. Micro RNA-19a suppresses thrombospondin-1 in CD35+ B cells in the intestine of mice with food allergy. Am J Transl Res 2016; 8: 5503-5511.
- Liu ZQ, Yang G, Geng XR, et al. Micro RNA-17-92 cluster mediates interleukin-4-suppressed IL-10 expression in B cells. Am J Transl Res 2016; 8: 2317-2324.
- Wang S, Tang Y, Cui H, et al. Let-7/miR-98 regulate Fas and Fas-mediated apoptosis. Genes Immun 2011; 12: 149-154. https://doi.org/10.1038/gene.2010.53
- Xie L, Xu J. Role of MiR-98 and ıts underlying mechanisms in systemic lupus erythematosus. J Rheumatol 2018; 45: 1397-1405. https://doi.org/10.3899/jrheum.171290
- Xie RD, Xu LZ, Yang LT, et al. Galectin-1 inhibits oral-intestinal allergy syndrome. Oncotarget 2017; 8: 13214-13222. https://doi.org/10.18632/oncotarget.14571
- Luo XQ, Yang SB, Qiu SQ, et al. Post-transcriptional regulation of interleukin-10 in peripheral B cells of airway allergy patients. Am J Transl Res 2016; 8: 5766-5772.
- Chen L, Xu J, Chu X, Ju C. MicroRNA-98 interferes with thrombospondin 1 expression in peripheral B cells of patients with asthma. Biosci Rep 2017; 37: BSR20170149. https://doi.org/10.1042/BSR20170149
- Biswas S, Thomas AA, Chen S, et al. MALAT1: an epigenetic regulator of ınflammation in diabetic retinopathy. Sci Rep 2018; 8: 6526. https://doi.org/10.1038/s41598-018-24907-w
- Hewitson JP, West KA, James KR, et al. MALAT1 suppresses immunity to infection through promoting expression of maf and IL-10 in Th cells. J Immunol 2020; 204: 2949-2960. https://doi.org/10.4049/jimmunol.1900940
- Hadjicharalambous MR, Lindsay MA. Long non-coding RNAs and the innate immune response. Noncoding RNA 2019; 5: 34. https://doi.org/10.3390/ncrna5020034
- Hu TJ, Huang HB, Shen HB, Chen W, Yang ZH. Role of long non-coding RNA MALAT1 in chronic obstructive pulmonary disease. Exp Ther Med 2020; 20: 2691-2697. https://doi.org/10.3892/etm.2020.8996
- Qiu YY, Wu Y, Lin MJ, Bian T, Xiao YL, Qin C. LncRNA-MEG3 functions as a competing endogenous RNA to regulate Treg/Th17 balance in patients with asthma by targeting microRNA-17/ RORγt. Biomed Pharmacother 2019; 111: 386-394. https://doi.org/10.1016/j.biopha.2018.12.080
- Fan M, Song W, Hao Z, Zhang J, Li Y, Fu J. Construction of lncRNA-miRNA-mRNA regulatory network in severe asthmatic bronchial epithelial cells: A bioinformatics study. Medicine (Baltimore) 2023; 102: e34749. https://doi.org/10.1097/MD.0000000000034749
- Yu HH, Zhao W, Zhang BX, Wang Y, Li J, Fang YF. Morinda officinalis extract exhibits protective effects against atopic dermatitis by regulating the MALAT1/miR-590-5p/CCR7 axis. J Cosmet Dermatol 2023; 22: 1602-1612. https://doi.org/10.1111/jocd.15610
- Zhao W, Yu HH, Meng WW, et al. Icariin restrains NLRP3 inflammasome-mediated Th2 immune responses and ameliorates atopic dermatitis through modulating a novel lncRNA MALAT1/miR-124-3p axis. Pharm Biol 2023; 61: 1249-1259. https://doi.org/10.1080/13880209.2023.2244004
- Feng H, Xiong X, Chen Z, Luo N, Wu Y. MALAT1 induces food allergy by promoting release of IL-6 from dendritic cells and suppressing the immunomodulatory function of tregs. J Asthma Allergy 2022; 15: 529-544. https://doi.org/10.2147/JAA.S341742
- Tirado-Rodriguez B, Ortega E, Segura-Medina P, Huerta-Yepez S. TGF- β: an important mediator of allergic disease and a molecule with dual activity in cancer development. J Immunol Res 2014; 2014: 318481. https://doi.org/10.1155/2014/318481
- Davoodi A, Lotfi R, Mortazavi SH, Gorgin Karaji A, Rezaiemanesh A, Salari F. Retinoic acid correlates with reduced serum IL-10 and TGF-β in allergic rhinitis. Rep Biochem Mol Biol 2021; 9: 399-407. https://doi.org/10.52547/rbmb.9.4.399
- Rosas A, Valencia MP, Sánchez M, Bautista M, Rico G, Vega GB. Transforming growth factor beta and platelets in allergic rhinitis and sinusitis. Rev Alerg Mex 2011; 58: 93-98.
- Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med 1999; 160: 216-220. https://doi.org/10.1164/ajrccm.160.1.9809140
- Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest 2009; 135: 66-73. https://doi.org/10.1378/chest.08-0722
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















