Graphical Abstract
Abstract
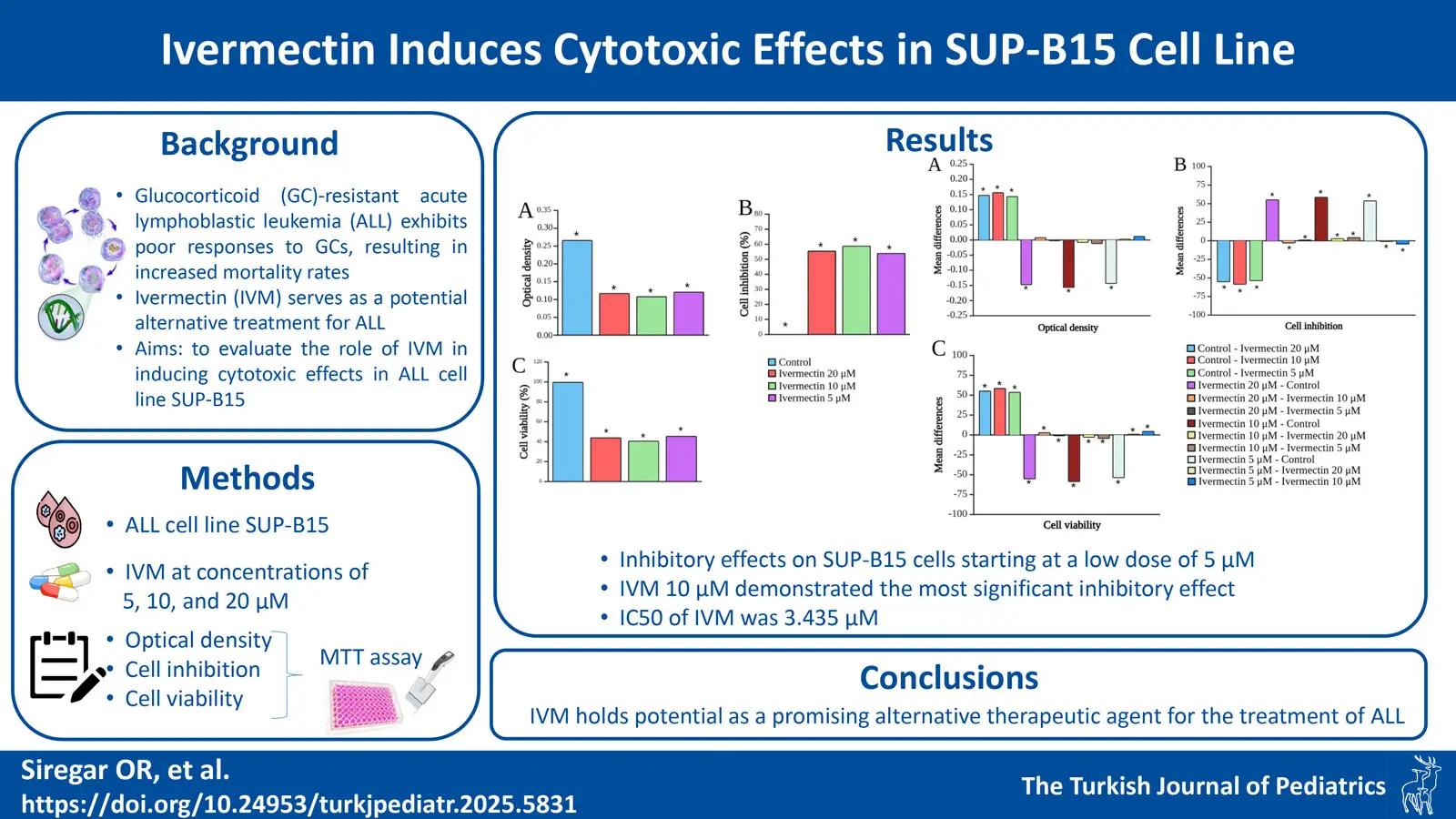
Background. Glucocorticoids remain the primary treatment for acute lymphoblastic leukemia (ALL) in children. However, glucocorticoid-resistant ALL exhibits increased mortality rates. To overcome resistance and improve management strategies, alternative therapeutic agents are required. Ivermectin (IVM), widely used as an anthelmintic agent, has been reported to possess anticancer properties through various mechanisms. These properties suggest IVM as a potential alternative treatment for ALL. This study aims to evaluate the role of IVM in inducing cytotoxic effects in the ALL cell line SUP-B15.
Methods. The ALL cell line SUP-B15 was examined following treatment with IVM at concentrations of 5, 10, and 20 μM. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess cytotoxic effects of IVM, including measurements of optical density, cell inhibition, and cell viability.
Results. IVM demonstrated inhibitory effects on SUP-B15 cells starting at a low dose of 5 μM. The half maximal inhibitory concentration (IC50) of IVM was 5 µM, showing an inhibitory effect of 54.18 ± 0.03% (95% confidence interval: 54.11-54.25%; p < 0.001). Treatment with 10 μM IVM demonstrated the most significant inhibitory effect (59.05 ± 0.1%) and the lowest cell viability (40.95 ± 0.01%).
Conclusions. IVM holds potential as a promising alternative therapeutic agent for the treatment of ALL.
Keywords: acute lymphoblastic leukemia, apoptosis, ivermectin, glucocorticoid resistance
Introduction
Acute lymphoblastic leukemia (ALL) is a malignancy of lymphoblasts, characterized by their uncontrolled and abnormal proliferation, which disrupts bone marrow production. This pathological process leads to the replacement of healthy bone marrow with leukemic cells, resulting in clinical manifestations associated with anemia, neutropenia, and thrombocytopenia.1 In 2020, more than 60,000 new leukemia cases and over 25,000 leukemia-related deaths were reported.2 ALL is the most reported type of leukemia, accounting for 70% of pediatric leukemia cases.3 The condition predominantly affects children under 15 years, with the highest incidence observed between ages 1 and 4.4 The 5-year survival rate of ALL is high in high-income countries, reported to be over 90%. Nevertheless, in low- and middle-income countries, this figure is substantially lower, where overall survival and disease-free survival rates are reported at 69.9% and 67.8%, respectively.3 Despite the high survival rate of ALL, numerous cases have reported poor prognoses influenced by treatment-related factors.5
Chemotherapy remains the first-line treatment for ALL, aiming to achieve remission through induction, intensification, and maintenance phases.6 The treatment regimen incorporates a range of cytostatic agents, with glucocorticoids (GCs) recognized as the most effective in inhibiting proliferation and inducing apoptosis in malignant cells.6,7 Currently, GCs, particularly prednisone and dexamethasone, are the primary therapeutic agents for the majority of lymphoid neoplasms in both pediatric and adult populations. GCs predominantly impact lymphoid tissue by inhibiting cell proliferation and inducing apoptosis.8 The cytotoxic properties of GCs have shown effectiveness in the treatment of ALL. Nonetheless, the emergence of GC resistance, observed both in vitro and in vivo, has been identified as a negative prognostic indicator in ALL.9
Resistance to GC has been a challenge in the management of ALL, as it results in inadequate treatment responses.10 Consequently, the risk of relapse increases, necessitating higher chemotherapy dosages and potentially resulting in severe adverse effects.11 Various studies have investigated alternative therapeutic strategies to manage GC resistance. One such potential alternative is ivermectin (IVM), which has been reported to exhibit anticancer properties through various mechanisms.12 One mechanism involves the induction of mitochondrial dysfunction and oxidative stress, which promotes increased apoptosis of leukemia cells, particularly in acute and chronic myeloid leukemia.13,14 In a leukemia xenograft model, IVM was shown to induce apoptosis and inhibit cell proliferation by enhancing the cleavage of poly(ADP-ribose) polymerase (PARP) and caspases.15 Another study also reported the efficacy of IVM in promoting apoptosis in esophageal cancer cells by elevating the BAX/BCL-2 ratio.16
This study aimed to investigate the role of IVM in inducing cytotoxic effects in the ALL cell line SUP-B15.
Materials and Methods
This study was approved by the Research Ethical Committee of the Faculty of Medicine, Universitas Sumatera Utara (approval number: 665/KEPK/USU/2023) and conducted according to the principles outlined in the Declaration of Helsinki. In this study, we examined the cytotoxic effects of IVM against SUP-B15 cells. The SUP-B15 cells were B lymphoblast cells derived from the bone marrow of an 8-year-old Caucasian male patient with ALL.
Cell cultures
SUP-B15 cells (ATCC, Manassas, USA) were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (GIBCO, Berlin, Germany), 2 mM L-glutamine, 50 µg/mL streptomycin, and 50 µg/mL penicillin in an incubator set at 37 °C and 5% CO2 concentration. The culture process was carried out in 24-well plates. To regulate confounding factors, including variations in culture conditions, this study followed a standard protocol of cell culturing and the cell culture was considered successful when the cell density ranged from 1.5 to 3 × 106 cells/mL.
Measurement of cytotoxic activity
Cytotoxic activity was measured in SUP-B15 cells by assessing optical density, inhibition, and cell viability using the 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay. The study was conducted in accordance with a standard protocol to mitigate confounding factors that affect cell viability, including the duration of the incubation period and the density of cells per well. The cells were categorized into 4 experimental groups: (1) IVM 5 µM; (2) IVM 10 µM; (3) IVM 20 µM; (4) negative controls. SUP-B15 cells were seeded in 96-well plates at a density of 2 × 104 cells/well and incubated at 37 °C under 5% CO2. After 24 hours of incubation, the cells were treated with IVM (5, 10, and 20 μM). Negative controls were obtained by excluding treatment from the wells. After 72 hours of treatment, the MTT assay was performed by adding 10 μL of MTT (5 mg/mL) to each well. Following a 4-hour incubation at 37 °C, absorbance was measured at a wavelength of 595 nm.
Statistical analysis
Data were processed and analyzed using the Statistical Product Service Solution (SPSS) software for Windows. The data were subjected to the Shapiro–Wilk normality test. A Levene’s test for homogeneity of variance was performed to assess the variability of the data. One-way analysis of variance (ANOVA) was performed to determine the relationship between the variables tested, followed by a post-hoc test. A p-value <0.05 was considered statistically significant.
Results
IVM exhibits cytotoxic effects on SUP-B15 cells
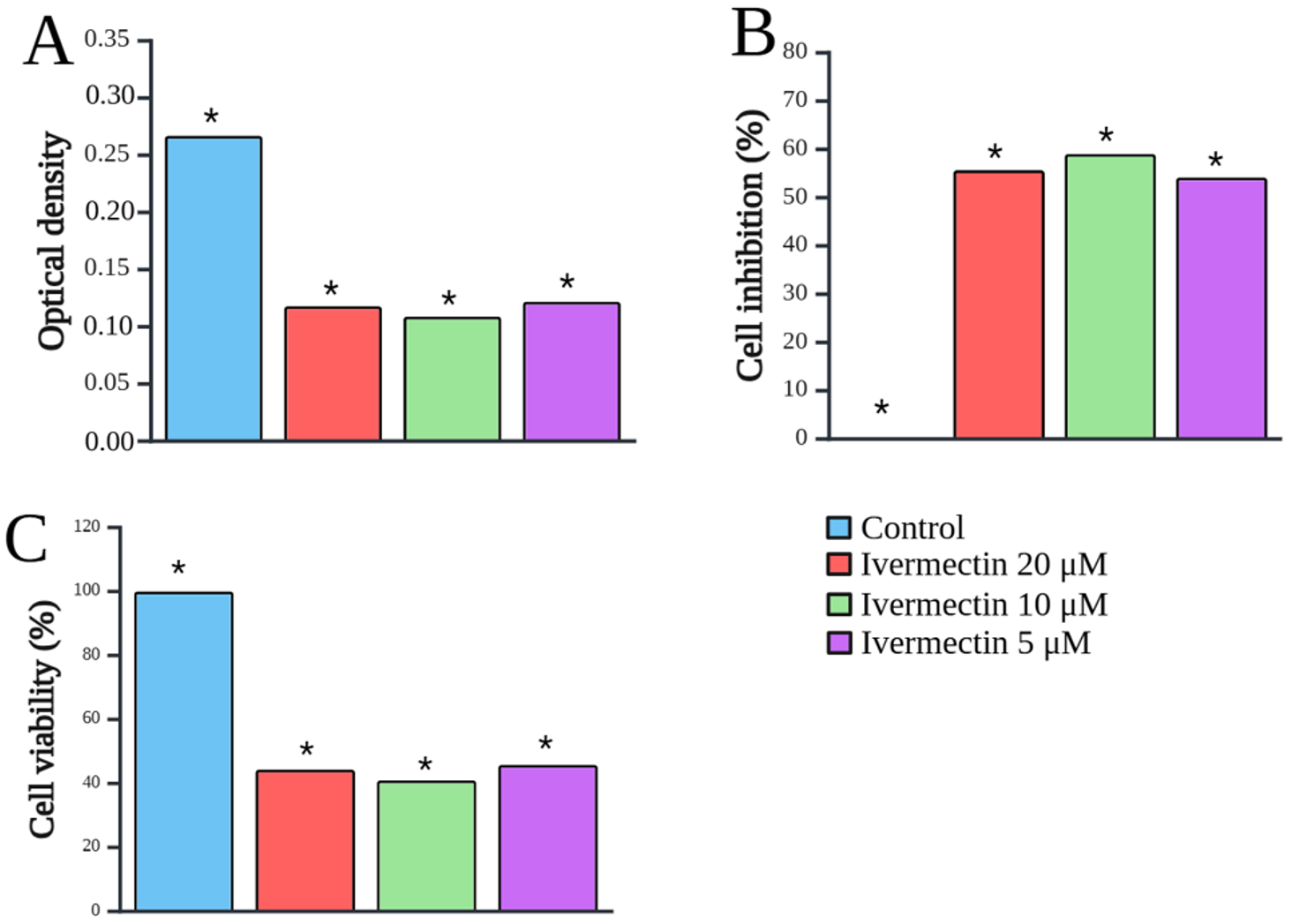
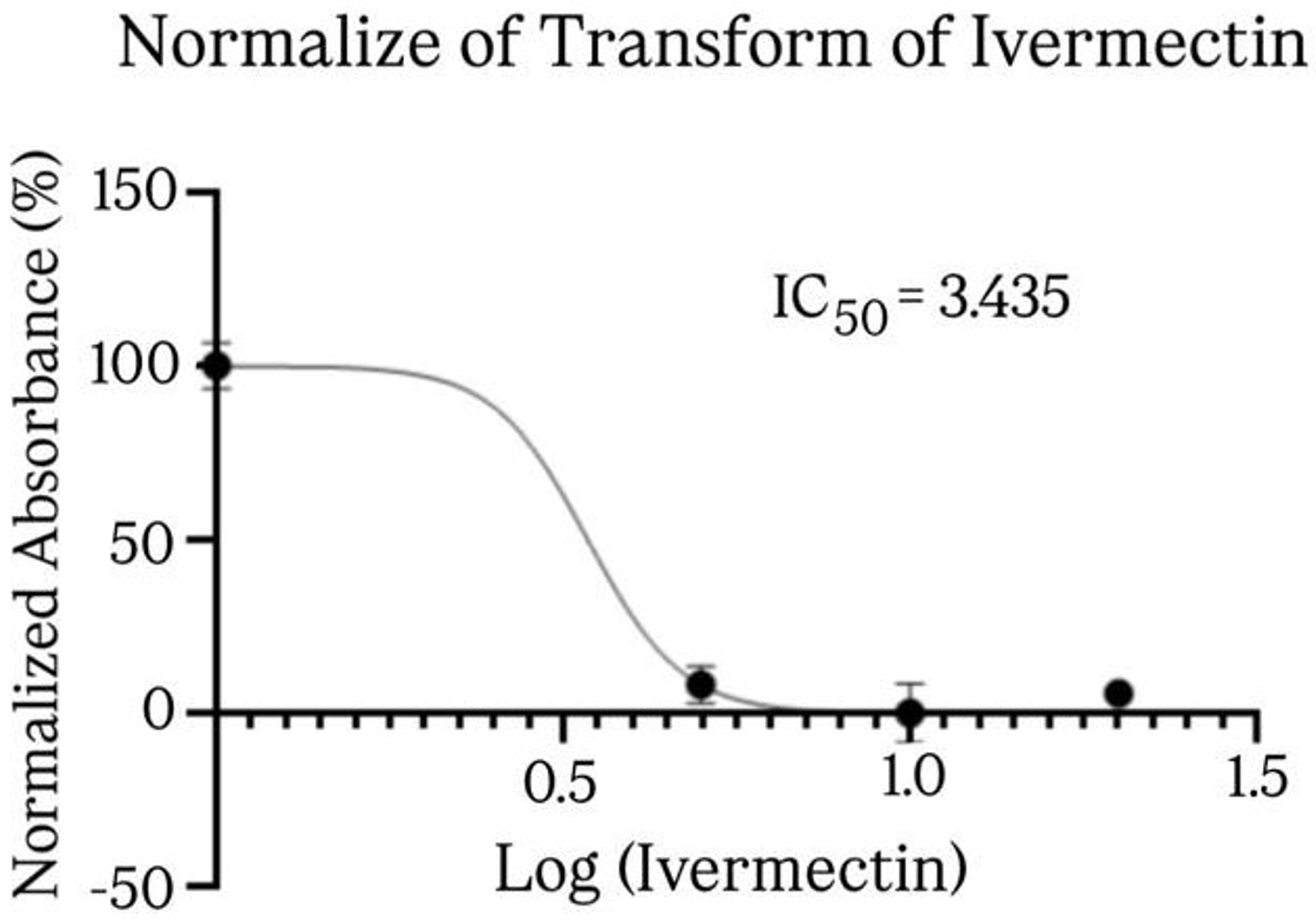
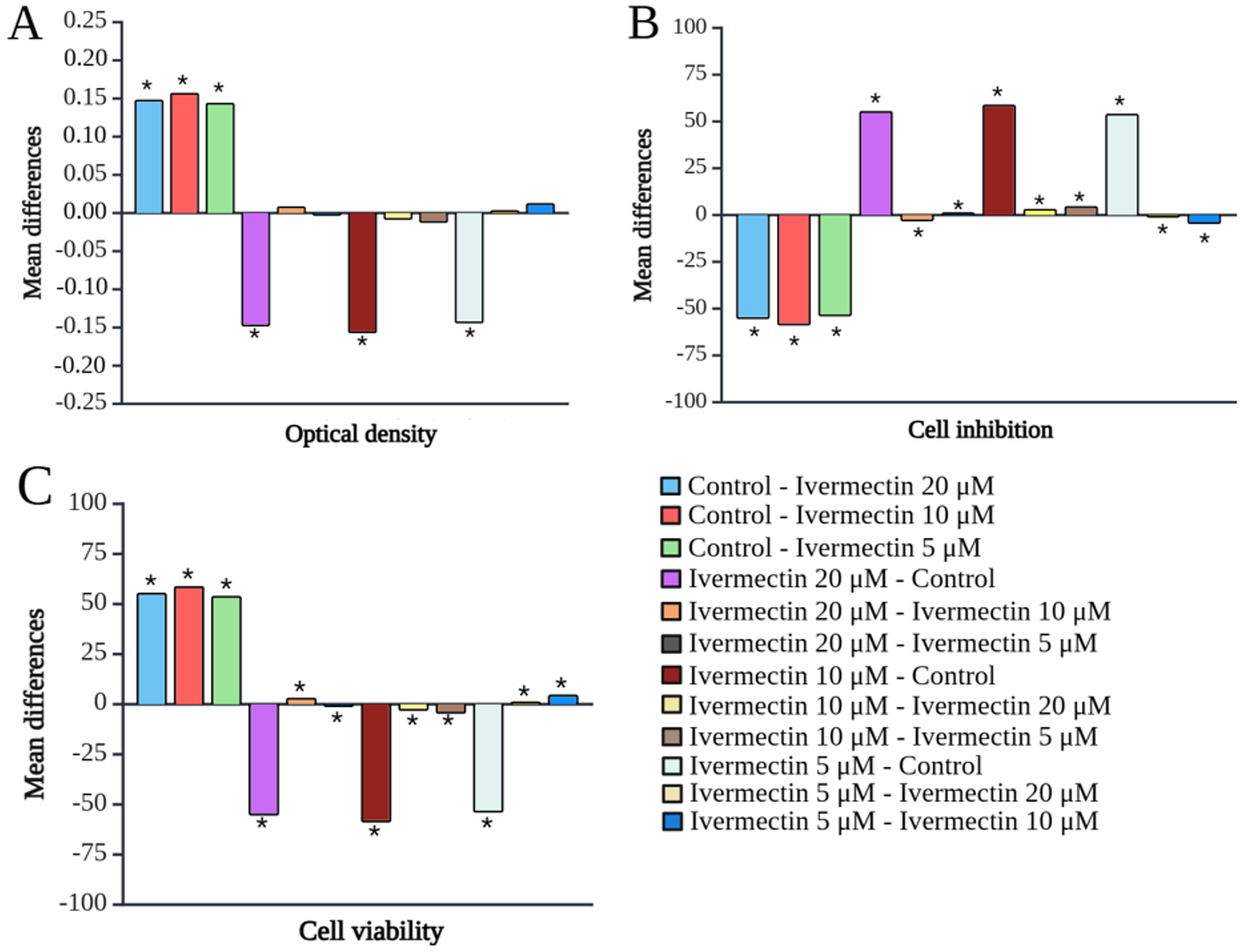
The cytotoxicity of IVM on SUP-B15 cells is shown in Fig. 1. IVM exerted a significant inhibitory effect on SUP-B15 cells compared to the control, starting from a low dose of 5 µM. Fig. 2 shows the IVM dose-response curve of the SUP-B15 cell viability, normalized according to the absorbance of the MTT assay data. The half maximal inhibitory concentration (IC50) of IVM was 3.435 µM. The curve took the form of a sigmoidal curve of the logarithmic inhibition model, which indicated increasing IVM concentrations caused a significant decrease in cell viability until reaching a plateau point approaching 0%. IVM at 10 µM produced the strongest inhibitory effect at 59.05 ± 0.10% (95% confidence interval [CI]: 58.80-59.29%; p < 0.001) and the lowest cell viability at 40.95 ± 0.01% (95% CI: 40.93-40.97%; p < 0.001). Fig. 3 presents the results of the post-hoc Bonferroni test. Optical density measurements yielded a p-value of <0.001 for the correlation between the IVM and control groups. However, no significant differences were observed among IVM doses (5, 10, and 20 µM) in the optical density test. All treatment correlations yielded a p-value <0.001 for inhibition and viability, indicating significant differences between all treatment groups and the control in both assessments.
Discussion
The results of this study demonstrated that IVM exhibited cytotoxic effects on SUP-B15 cells in vitro, with observable effects starting at a low dose of 5 µM. These findings are consistent with a previous study that reported IVM to inhibit the proliferation of colorectal cancer cells at a similar dose of 5 µM.17 IVM has been reported to exert an inhibitory effect on malignant cells through various mechanisms.12 Liu et al. reported that IVM inhibited malignant cell proliferation by inducing autophagy and apoptosis.18 Furthermore, IVM induced apoptosis and delayed the proliferation of malignant cells by increasing the cleavage of PARP and caspases.15 Another study reported that IVM can induce cell apoptosis even in the absence of receptor-interacting serine/threonine protein kinase 1 (RIPK1) and effector caspases, further supporting its potential as a potent anticancer drug.19 Xu et al. also demonstrated that IVM significantly induced reactive oxygen species (ROS) accumulation, inhibited the activation of the NF-κB signaling pathway, and elevated the BAX/BCL-2 ratio, thereby effectively reducing the proliferation of esophageal cancer cells.16 Elevated BAX and caspase-3 expressions, along with reduced BCL-2 expression, are known to promote cell apoptosis, ultimately inhibiting the proliferation of ALL cells.20,21
In this study, IVM at 10 µM concentration was reported to yield the strongest inhibitory effect on SUP-B15 cells. This finding contrasts with the pharmacokinetics of IVM, which typically exhibit antiparasitic and antiviral effects in a dose-dependent manner.22,23 Interestingly, 10 µM IVM was more cytotoxic than 20 µM because the cytotoxic effects were exhibited through the induction of oxidative stress.14 While oxidative stress triggered apoptosis in malignant cells, it also initiated and promoted the development of malignant cells. Excessive doses of IVM can result in a significant accumulation of oxidative stress, potentially contributing to the initiation and proliferation of malignant cells.24 Therefore, a concentration of 10 µM IVM was determined as the optimal dose to demonstrate cytotoxic effects in leukemia.
Limitations and suggestions for further research
A limitation of this study was the utilization of a single cell line due to budgetary limitations. The utilization of a single cell line limited the generalizability of IVM’s efficacy on ALL. Research involving several cell lines and studies comparing IVM’s efficacy against standard ALL treatments such as GCs or chemotherapeutic agents could provide better evidences regarding the efficacy of IVM on ALL. Further investigation of cell cycle alterations is necessary to improve our understanding of the apoptosis induced by IVM. Additional in vivo or an animal model study is recommended for better clinical applicability. This study provides a basis for subsequent in vitro investigations into the role of IVM in inducing cytotoxic effects in ALL.
Conclusions
IVM is increasingly recognized for its diverse therapeutic functions beyond its established antiparasitic properties. The cytotoxic effects of IVM against ALL were demonstrated in this study, and the findings suggest that IVM holds potential as an alternative therapeutic approach for ALL.
Ethical approval
The study was approved by the Research Ethical Committee of the Faculty of Medicine, Universitas Sumatera Utara (clearance number: 665/KEPK/USU/2023) and was conducted according to the Declaration of Helsinki.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Puckett Y, Chan O. Acute lymphocytic leukemia. Treasure Island (FL): StatPearls Publishing. 2025. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459149/ (Accessed on Dec, 2024).
- Mohammadian-Hafshejani A, Farber IM, Kheiri S. Global incidence and mortality of childhood leukemia and its relationship with the Human Development Index. PLoS One 2024; 19: e0304354. https://doi.org/10.1371/journal.pone.0304354
- Ahmad I, Ghafoor T, Ullah A, et al. Pediatric acute lymphoblastic leukemia: clinical characteristics, treatment outcomes, and prognostic factors: 10 years’ experience from a low- and middle-income country. JCO Glob Oncol 2023; 9: e2200288. https://doi.org/10.1200/GO.22.00288
- Miranda-Filho A, Piñeros M, Ferlay J, Soerjomataram I, Monnereau A, Bray F. Epidemiological patterns of leukaemia in 184 countries: a population-based study. Lancet Haematol 2018; 5: e14-e24. https://doi.org/10.1016/S2352-3026(17)30232-6
- Ramadhan MH, Sari NM, Peryoga SU, Susanah S. Survival and treatment outcomes of childhood acute lymphoblastic leukemia in a low-middle income country: a single-center experience in west java, Indonesia. J Blood Med 2024; 15: 77-85. https://doi.org/10.2147/JBM.S438042
- Garbim MR, Broto GE, Trigo FC, et al. Chemotherapy induces plasmatic antioxidant changes in pediatric patients with acute lymphoid leukemia B that correlate to disease prognosis. Curr Res Immunol 2022; 3: 228-233. https://doi.org/10.1016/j.crimmu.2022.09.001
- Amjad MT, Chidharla A, Kasi A. Cancer chemotherapy. Treasure Island (FL): StatPearls Publishing. 2025. Available at: https://www.ncbi.nlm.nih.gov/books/NBK564367/ (Accessed on Dec, 2024).
- Pourhassan H, Murphy L, Aldoss I. Glucocorticoid therapy in acute lymphoblastic leukemia: navigating short-term and long-term effects and optimal regimen selection. Curr Hematol Malig Rep 2024; 19: 175-185. https://doi.org/10.1007/s11899-024-00735-w
- Borin C, Pieters T, Serafin V, Ntziachristos P. Emerging epigenetic and posttranslational mechanisms controlling resistance to glucocorticoids in acute lymphoblastic leukemia. Hemasphere 2023; 7: e916. https://doi.org/10.1097/HS9.0000000000000916
- Sciarrillo R, Wojtuszkiewicz A, Kooi IE, et al. Glucocorticoid resistant pediatric acute lymphoblastic leukemia samples display altered splicing profile and vulnerability to spliceosome modulation. Cancers (Basel) 2020; 12: 723. https://doi.org/10.3390/cancers12030723
- Sarno J, Domizi P, Liu Y, et al. Dasatinib overcomes glucocorticoid resistance in B-cell acute lymphoblastic leukemia. Nat Commun 2023; 14: 2935. https://doi.org/10.1038/s41467-023-38456-y
- Juarez M, Schcolnik-Cabrera A, Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am J Cancer Res 2018; 8: 317-331.
- Sharmeen S, Skrtic M, Sukhai MA, et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood 2010; 116: 3593-3603. https://doi.org/10.1182/blood-2010-01-262675
- Wang J, Xu Y, Wan H, Hu J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem Biophys Res Commun 2018; 497: 241-247. https://doi.org/10.1016/j.bbrc.2018.02.063
- Ishikawa C, Senba M, Mori N. Importin β1 regulates cell growth and survival during adult T cell leukemia/lymphoma therapy. Invest New Drugs 2021; 39: 317-329. https://doi.org/10.1007/s10637-020-01007-z
- Xu N, Lu M, Wang J, et al. Ivermectin induces apoptosis of esophageal squamous cell carcinoma via mitochondrial pathway. BMC Cancer 2021; 21: 1307. https://doi.org/10.1186/s12885-021-09021-x
- Zhou S, Wu H, Ning W, et al. Ivermectin has new application in inhibiting colorectal cancer cell growth. Front Pharmacol 2021; 12: 717529. https://doi.org/10.3389/fphar.2021.717529
- Liu J, Zhang K, Cheng L, Zhu H, Xu T. Progress in understanding the molecular mechanisms underlying the antitumour effects of ivermectin. Drug Des Devel Ther 2020; 14: 285-296. https://doi.org/10.2147/DDDT.S237393
- Mezzatesta C, Abduli L, Guinot A, et al. Repurposing anthelmintic agents to eradicate resistant leukemia. Blood Cancer J 2020; 10: 72. https://doi.org/10.1038/s41408-020-0339-9
- Majtnerová P, Roušar T. An overview of apoptosis assays detecting DNA fragmentation. Mol Biol Rep 2018; 45: 1469-1478. https://doi.org/10.1007/s11033-018-4258-9
- McKenna S, García-Gutiérrez L, Matallanas D, Fey D. BAX and SMAC regulate bistable properties of the apoptotic caspase system. Sci Rep 2021; 11: 3272. https://doi.org/10.1038/s41598-021-82215-2
- Schulz JD, Coulibaly JT, Schindler C, Wimmersberger D, Keiser J. Pharmacokinetics of ascending doses of ivermectin in Trichuris trichiura-infected children aged 2-12 years. J Antimicrob Chemother 2019; 74: 1642-1647. https://doi.org/10.1093/jac/dkz083
- Sabry N, Fouad Y, AbdAllah M, Cordie A, Esmat G. Dose-dependent ivermectin effect on COVID-19 polymerase chain reaction status. Am J Ther 2024; 31: e72-e81. https://doi.org/10.1097/MJT.0000000000001490
- Leone A, Roca MS, Ciardiello C, Costantini S, Budillon A. Oxidative stress gene expression profile correlates with Cancer patient poor prognosis: identification of crucial pathways might select novel therapeutic approaches. Oxid Med Cell Longev 2017; 2017: 2597581. https://doi.org/10.1155/2017/2597581
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.