Graphical Abstract
Abstract
Background. The expression and clinical correlation of BRAFV600E mutation and programmed cell death-1 ligand 1 (PD-L1) in children with Langerhans cell histiocytosis (LCH) have been reported, but the conclusions of previous studies are inconsistent. In addition, it has been reported that elevated cathepsin S (CTSS) expression is associated with various cancers. However, there is currently no research on the correlation between CTSS and LCH. The aim of this study was to reassess the clinical correlation of BRAFV600E mutation and PD-L1 in pediatric LCH and to investigate the expression and clinical correlation of CTSS in children with LCH.
Methods. A total of 35 tissue samples were analyzed for the BRAFV600E gene mutation using droplet digital polymerase chain reaction, and 31 tissue samples were examined for CTSS and PD-L1 by immunohistochemistry. In addition, the clinical characteristics and prognosis of these 35 pediatric patients were analyzed and summarized.
Results. The incidence of BRAFV600E gene mutation was 34.3% (12/35). The occurrence of BRAFV600E gene mutation was significantly associated with age ≤ 2 years and involvement of central nervous system risk sites (66.7% and 72.7%, respectively). The expression rate of PD-L1 was 35.5% (11/31), and it was significantly correlated with cutaneous involvement (100%, 3/3). PD-L1 expression was unrelated to BRAFV600E gene mutation. Neither BRAFV600E gene mutation nor PD-L1 expression had a significant impact on disease progression/reactivation and initial 6-week treatment response. CTSS was expressed positively in the lesion tissues of all 31 children with LCH. The H-scores of CTSS were significantly associated with age ≤ 2 years. CTSS had no significant effect on the initial 6-week treatment response, disease progression/reactivation, BRAFV600E gene mutation, or PD-L1 expression.
Conclusions. CTSS is positively expressed in LCH, and its expression level is associated with onset age ≤ 2 years. BRAFV600E gene mutation, PD-L1, and CTSS may not be associated with the prognosis of LCH.
Keywords: Langerhans cell histiocytosis, cathepsin S, BRAFV600E, programmed cell death-1 ligand 1 (PD-L1)
Introduction
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasm1, which affects children at an annual rate of approximately 3-9 per million2 and adults at a rate of approximately 1-2 per million.3 The clinical manifestations of LCH exhibit high heterogeneity, involving various systems throughout the body, ranging from spontaneously regressing isolated bone lesions or skin lesions to multisystem disease with involvement of risk organs (RO+) that can be life-threatening. The 5-year overall survival rate has reached over 80%, but the incidence of disease progression/reactivation still ranges between 30 and 50%.4-6 Currently, LCH is believed to be a myeloid neoplasm driven by abnormal activation of the mitogen-activated protein kinase pathway.7 The most common mutated gene in this signaling pathway is BRAFV600E, with an incidence of 36-60%.8-12 In addition, inhibitors of the mitogen-activated protein kinase pathway (dabrafenib and trametinib) are effective and safe for LCH.13
The correlation between BRAFV600E mutation and clinical features, treatment response, and clinical outcomes of LCH that has been reported in different studies is inconsistent. An international cohort study12 reported an association between BRAFV600E and younger age at diagnosis and a higher prevalence of multisystem involvement, high-risk disease, and cutaneous involvement. In the entire cohort, BRAFV600E was associated with decreased event-free survival rates. A study9 conducted in Türkiye also demonstrated that BRAFV600E mutation was significantly associated with multisystem involvement, younger age (< 2 years), and cutaneous or special organ involvement, and was an independent predictor of disease recurrence. However, research conducted in Japan revealed that the BRAFV600E mutation showed no correlation with gender, age at diagnosis, disease severity, response to frontline treatment, recurrence, or sequelae related to central nervous system (CNS).8
Immune checkpoint programmed cell death-1 and its ligand programmed cell death-1 ligand 1 (PD-L1) are associated with the pathogenesis of various malignancies. The positive expression rate of PD-L1 in LCH was reported to be 32%, and increased PD-L1 expression acted as an independent predictor of poor disease-free survival.14 However, Tandon et al.15 reported no significant correlation between PD-L1 expression and clinical outcomes of childhood LCH. Therefore, further research is needed to investigate the correlation of PD-L1 expression with clinical features, treatment response, and clinical outcomes of LCH.
Thus far, no biomarkers for risk stratification have been identified in LCH.1 It is necessary to actively seek new tumor biomarkers to improve risk stratification, identify potential molecular targeted therapies, and reduce the incidence of disease reactivation and sequelae (such as diabetes insipidus, growth retardation, sclerosing cholangitis, etc.).
Recently, it has been reported that increased cathepsin S (CTSS) expression is associated with different types of cancers.16,17 CTSS is a papain-type cysteine protease that is widely present in various cells.18 Typically, the secretion of CTSS occurs via vesicle exocytosis induced by elevated intracellular Ca2+ levels, resulting in lysosome fusion with the plasma membrane and subsequent release of their contents into the extracellular space.19 CTSS plays roles in antigen presentation, cell signaling transduction, and the promotion of chemokine or cytokine release.20 It is also involved in regulating multiple pathophysiological processes, including immune response modulation, angiogenesis and remodeling, as well as the promotion of tumor cell proliferation and metastasis.18 The hydrolytic cleavage of specific proteins by CTSS contributes to pro-tumorigenic conditions, influences signaling pathways, and promotes tumor cell metastasis.16 However, to the best of our knowledge no studies have yet explored the relationship between CTSS and LCH. Therefore, we hypothesize that there is a relationship between CTSS and LCH.
Herein, this study reassessed the expression and clinical correlation of BRAFV600E mutations and PD-L1 in children with LCH, meanwhile, investigating the expression of CTSS in pediatric LCH and its relationship with the clinical characteristics, treatment response, and prognosis of LCH.
Materials and Methods
Patients
A total of 35 children with LCH admitted for inpatient treatment at the Department of Pediatric Hematology and Oncology, the Affiliated Hospital of Qingdao University from January 1, 2018, to September 30, 2023, were selected for the study. The diagnosis of LCH relied on the pathological biopsy results of affected tissues, with confirmation by immunohistochemical staining for CD1a and CD207 (Langerin) positivity.7 Patients were classified into single-system involvement and multisystem involvement based on the number of affected systems. Risk organs (RO) included the hematopoietic system / bone marrow, liver, and spleen.6,7 CNS-risk sites included craniofacial, ocular, auricular, and oral regions.6,7 This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Qingdao University and complied with the requirements of the Helsinki Declaration revised in 2013, with informed consent obtained from the guardians of the children.
LCH treatment regimens and assessment criteria for treatment response
Treatment for all patients with LCH followed a stratified approach based on clinical risk, and the chemotherapy regimen was LCH-III.7 Targeted therapy with dabrafenib (initiated during the induction phase and continued throughout the entire treatment course) was added to the treatment for patients with BRAFV600E mutation. The chemotherapy regimen for patients with disease progression/reactivation was the BCH-LCH 2014 protocol (a combination of cytarabine, vindesine, and dexamethasone).6 Treatment response was categorized as follows: 1) nonactive disease, 2) active disease/better (AD-better), 3) AD/intermediate (AD-intermediate), and 4) AD/worse (AD-worse).7 Response to treatment included nonactive disease and AD-better. Disease progression comprised AD-intermediate and AD-worse. Reactivation was defined as the reappearance of signs and symptoms of active disease after complete remission or after a disease control period lasting more than 3 months following maintenance therapy.6
Droplet digital polymerase chain reaction (DDPCR) for BRAFV600E mutation detection
The BRAFV600E mutation was detected by DDPCR (the most sensitive method21). Tissue DNA was extracted from unstained paraffin-embedded tissue at diagnosis using the GeneRead DNA FFPE Kit (180134, QIAGEN, Hilden, Germany). BRAFV600E mutation detection was performed using the DDPCR System (SG-2000, Suzhou RainSure Scientific CO., LTD., Suzhou, Jiangsu, China). DDPCR-related reagents were purchased from Suzhou RainSure Scientific CO., LTD. The reaction program included a pre-denaturation step at 95 °C for 10 min, followed by 40 amplification cycles of 95.3 °C for 30 s and 57 °C for 1 min, and a final extension at 98 °C for 10 min, with a holding step at 20 °C for 2 min. The detection limit was set at 0.1%. Primers and probes were designed using AlleleID (version 6.0, PREMIER Biosoft, Palo Alto, CA, USA) as follows:
Forward primer: 5’-TGCTTGCTCTGATAGGAAAATGA-3’
Reverse primer: 5’-CCATCCACAAAATGGATCCAGAC-3’
Wild-type probe: FAM-AGCTACAGTGAAATC-MGB
Mutant probe: VIC-AGCTACAGAGAAATCTC-MGB
Immunohistochemistry (IHC) for PD-L1 and CTSS
IHC was performe d on formalin-fixed paraffin-embedded tissue sections. Continuous formalin-fixed paraffin-embedded tissue sections (4-μm thick) were deparaffinized and hydrated. After heat-induced antigen retrieval, endogenous peroxidase was blocked with 3% H2O2. Non-specific antigens were blocked with 5% goat serum. The sections were then incubated with anti-PD-L1 rabbit monoclonal antibody (SP142, working solution, 08008540001, Roche Diagnostics GmbH, Tucson, AZ, USA) and rabbit monoclonal antibody against CTSS (EPR5128, 1:250, ab134157, Abcam, Cambridge, U.K.) at 37 °C for 60 min. Subsequently, the sections were incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:250, ab6721, Abcam, Cambridge, U.K.) at 37 °C for 30 min. After incubation, the sections were stained with diaminobenzidine chromogen solution (ab64238, Abcam, Cambridge, U.K.) and observed under an optical microscope. All experiments were repeated twice. Selected cases underwent dual IHC using the VECTASTAIN® ABC kit (PK-6200 and AK-5200, Vecort Laboratories, Burlingame, CA, USA) and substrate kits (diaminobenzidine substrate kit, SK-4100 and VECTOR Red Substrate kit, SK-5100, Vecort Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions for CTSS (ab134157, Abcam, Cambridge, U.K., 1:250) and Langerin (1:500, bs-2650R, Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) staining.
IHC was performed on tissue samples from 31 patients, as the remaining 4 patients had insufficient tissue samples. Immunohistochemical results were assessed by experienced pathologists. PD-L1 staining in > 5% of total cells was considered positive.22 A semi-quantitative scoring system using the H-score was applied. The staining percentage (0-100%) and intensity (0-3: 0, negative; 1, weak; 2, moderate; 3, strong) of CTSS in the lesions were evaluated, and the H-score was calculated using the following formula (0-300): H-score = (% of cells of weak intensity × 1) + (percentage of cells of moderate intensity × 2) + (percentage of cells of strong intensity × 3).23
Statistical analysis
Data analysis was performed using IBM SPSS Statistics (version 26, International Business Machines Corporation, Amonk, NY, USA). Continuous variables are expressed as mean ± standard deviation (x±s). For intergroup quantitative comparisons, t-test was used if the data met the normal distribution, and the Mann-Whitney U test was used if the normal distribution assumption was violated. Qualitative data are reported as percentages, and the Fisher’s exact test was employed. Multivariate analysis was conducted by logistic regression analysis. A p-value < 0.05 was considered statistically significant.
Results
General clinical features
Bone involvement (94.3%) was the most common. Multisystem involvement occurred in 65.7% of cases. RO involvement was observed in 22.9% of cases, with the liver being the most frequently affected organ (14.3%). Liver involvement mainly manifested as hepatomegaly, with two cases progressing to cholangitis (one with intrahepatic bile duct stones). BRAFV600E gene mutation was detected in 12 cases (34.3%), with 83.3% (10/12) of them receiving targeted therapy (dabrafenib). The overall rate of response to treatment for the 35 patients was 85.7%. The incidence of disease progression/reactivation was 14.3% (5/35). See Table I for basic clinical characteristics.
| CNS, central nervous system; HLH, hemophagocytic lymphohistiocytosis; LCH, Langerhans cell histiocytosis. | ||
| Table I. General clinical characteristics of 35 children with LCH. | ||
| Characteristics |
|
|
| Gender | ||
| Male |
|
|
| Female |
|
|
| Age (years) | ||
| ≤ 2 |
|
|
| > 2 |
|
|
| Involvement of sites | ||
| Skin |
|
|
| Bone |
|
|
| CNS-risk site |
|
|
| CNS |
|
|
| Liver |
|
|
| Spleen |
|
|
| Hematopoietic system/bone marrow |
|
|
| Lung |
|
|
| Lymph node |
|
|
| Endocrine system (pituitary) |
|
|
| Involvement of systems | ||
| Single system |
|
|
| Multisystem |
|
|
| Risk organ involvement | ||
| Yes |
|
|
| No |
|
|
| Complication: HLH |
|
|
| Targeted therapy (dabrafenib) |
|
|
| Response to treatment |
|
|
| Treatment with dabrafenib |
|
|
| Treatment without dabrafenib |
|
|
| Initial 6-week treatment response |
|
|
| Treatment with dabrafenib |
|
|
| Treatment without dabrafenib |
|
|
| Progress/reactivation |
|
|
| Risk organ involvement |
|
|
| Without risk organ involvement |
|
|
| Deaths |
|
|
Association of BRAFV600E gene mutation with clinical features, treatment response, and prognosis
The incidence of BRAFV600E gene mutation was 34.3% (12/35). The mutation rates in the ≤ 2 years age group and > 2 years age group were 66.7% and 17.4%, respectively, with a statistically significant difference (P < 0.05). The mutation rates in the CNS-risk site involvement group and non-CNS-risk site involvement group were 72.7% and 16.7%, respectively, with a statistically significant difference (P < 0.05). BRAFV600E gene mutation was not associated with gender, RO+, multisystem involvement, CNS involvement, bone involvement, skin involvement, lymph node involvement, pituitary involvement, initial 6-week treatment response, or disease progression/reactivation, with no statistically significant differences (P > 0.05); see Table II for details.
| Table II. The correlation between BRAFV600E gene mutations and clinical characteristics, treatment response, and prognosis in children with LCH (n=35). | |||
|---|---|---|---|
| Factors |
|
|
|
|
(+), mutation positive; (-), mutation negative CNS, central nervous system; LCH, Langerhans cell histiocytosis. * Fisher's exact test. |
|||
| Gender |
|
||
| Male |
|
|
|
| Female |
|
|
|
| Age (years) |
|
||
| ≤ 2 |
|
|
|
| > 2 |
|
|
|
| Risk organ involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Multisystem involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| CNS-risk site involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| CNS involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Bone involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Skin involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Lymph node involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Endocrine system (pituitary) involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Initial 6-week treatment response |
|
||
| Response |
|
|
|
| No response |
|
|
|
| Progress/reactivation |
|
||
| Yes |
|
|
|
| No |
|
|
|
Association of PD-L1 expression with clinical features, treatment response, and prognosis
The expression rate of PD-L1 was 35.5% (11/31). The expression rates in the skin involvement group and non-skin involvement group were 100% (3/3) and 28.6% (8/28), respectively, with a statistically significant difference (P = 0.037). PD-L1 expression showed no correlation with gender, age at onset, BRAFV600E gene mutation, RO+, multisystem involvement, CNS involvement, CNS-risk site involvement, bone involvement, lymph node involvement, pituitary involvement, initial 6-week treatment response, or disease progression/reactivation, with no statistically significant differences (P > 0.05); see Table III for details.
| Table III. The correlation between PD-L1 expression and clinical characteristics, treatment response, and prognosis in children with LCH (n=31). | |||
|---|---|---|---|
| Factors |
|
|
|
|
(+), expression positive; (-), expression negative; CNS, central nervous system;LCH, Langerhans cell histiocytosis; PD-L1, programmed cell death-1 ligand 1. * Fisher's exact test. |
|||
| Gender |
|
||
| Male |
|
|
|
| Female |
|
|
|
| Age (years) |
|
||
| ≤ 2 |
|
|
|
| > 2 |
|
|
|
| BRAFV600E gene mutation |
|
||
| (+) |
|
|
|
| (-) |
|
|
|
| Risk organ involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Multisystem involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| CNS-risk site involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| CNS involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Bone involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Skin involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Lymph node involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Endocrine system (pituitary) involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Initial 6-week treatment response |
|
||
| Response |
|
|
|
| No response |
|
|
|
| Progress/reactivation |
|
||
| Yes |
|
|
|
| No |
|
|
|
Relationship of CTSS expression with clinical features, treatment response, and prognosis
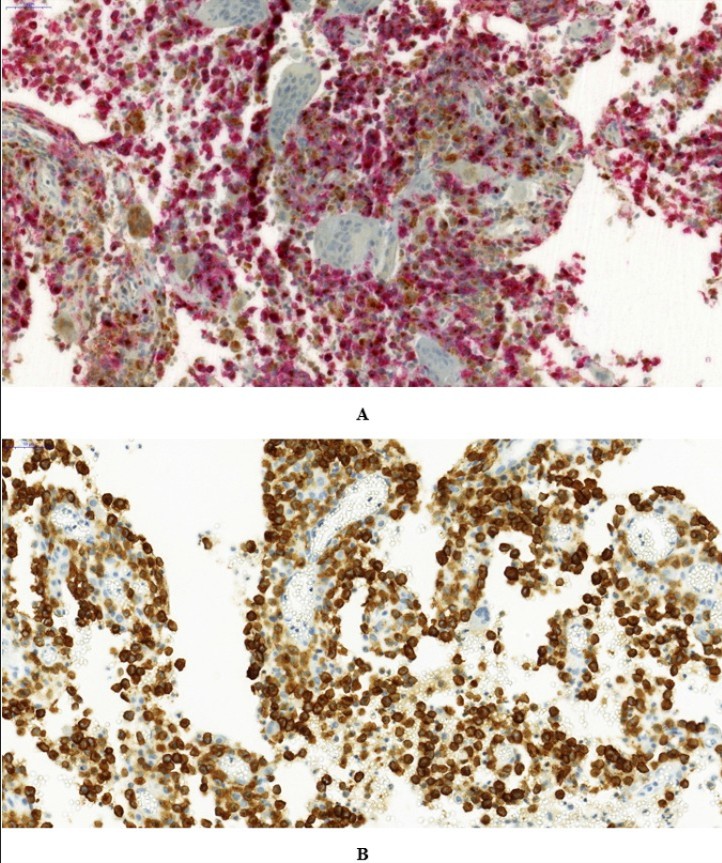
CTSS showed positive expression in tissues of all 31 children (Fig. 1). The expression levels of CTSS in the ≤ 2 years age group and > 2 years age group were 216.00 ± 35.65 and 169.05 ± 63.79, respectively, with a statistically significant difference (P < 0.05). CTSS expression showed no correlation with gender, multisystem involvement, RO+, CNS-risk site involvement, CNS involvement, bone involvement, skin involvement, lymph node involvement, pituitary involvement, initial 6-week treatment response, disease progression/reactivation, BRAFV600E gene mutation, or PD-L1 expression, with no statistically significant differences (P > 0.05, Table IV).
| Table IV. The relationship between CTSS expression and clinical characteristics, treatment response, and prognosis in children with LCH (n=31). | ||||
|---|---|---|---|---|
| Factors |
|
|
|
|
| LCH, Langerhans cell histiocytosis; CTSS, Cathepsin S; PD-L1, programmed cell death-1 ligand 1; CNS, central nervous system; (+), positive; (-), negative. | ||||
| Gender |
|
|
||
| Male |
|
|
||
| Female |
|
|
||
| Age (years) |
|
|
||
| ≤ 2 |
|
|
||
| > 2 |
|
|
||
| BRAFV600E gene mutation |
|
|
||
| Positive |
|
|
||
| Negative |
|
|
||
| PD-L1 expression |
|
|
||
| Positive |
|
|
||
| Negative |
|
|
||
| Risk organ involvement |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
| Multisystem involvement |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
| CNS-risk site involvement |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
| CNS involvement |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
| Skin involvement |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
| Lymph node involvement |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
| Endocrine system (pituitary) involvement |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
| Initial 6-week treatment response |
|
|
||
| Response |
|
|
||
| No response |
|
|
||
| Progress/reactivation |
|
|
||
| Yes |
|
|
||
| No |
|
|
||
Characteristics of children aged ≤ 2 years
The incidence of multisystem involvement in the ≤ 2 years age group and > 2 years age group was 91.7% and 52.2%, respectively (P < 0.05). The incidence of risk organ involvement in the ≤ 2 years age group and > 2 years age group was 58.3% and 4.3%, respectively (P < 0.05). The mutation rates of BRAFV600E in the ≤ 2 years age group and > 2 years age group were 66.7% and 17.4%, respectively (P < 0.05). The expression levels of CTSS in the ≤ 2 years age group and > 2 years age group were 216.00 ± 35.65 and 169.05 ± 63.79, respectively (P < 0.05), all exhibiting statistically significant differences (details presented in Table IV and TableV).
Multivariate analysis of the relationship of the prognosis (progression / reactivation) of LCH with CTSS, BRAFV600E gene mutations, and PD-L1
The results of the logistic regression analysis showed that CTSS expression, BRAFV600E gene mutations, and PD-L1 expression in tissue were not associated with the prognosis (progression/reactivation) of LCH, with no statistically significant differences (P>0.05).
Discussion
In our study, the incidence of the BRAFV600E gene mutation was 34.3%, and it was significantly associated with age ≤ 2 years and involvement of CNS risk sites. The positive expression rate of PD-L1 was 35.5%, and it was significantly correlated with cutaneous involvement (notably, only three children exhibited cutaneous involvement). CTSS was positively expressed in the lesion tissues of all children with LCH. The H-scores of CTSS were significantly associated with age ≤ 2 years.
The reported incidence of tissue BRAFV600E gene mutations vary among different research centers. The reported incidence of BRAFV600E gene mutations in China ranges from 30 to 60%, and the incidence in East Asian populations may be lower than that in other ethnic groups.10,11,14,24 In this study, the incidence of BRAFV600E gene mutations was 34.3%, which is consistent with the aforementioned research findings but lower than the results reported in an international cohort study (50.7%).12 This may be due to the preservation time of the specimen, the abundance of tumor tissue within it, or the relatively small sample size. BRAFV600E gene mutations were correlated with onset age ≤ 2 years, consistent with the findings of previous studies.24 Additionally, this study found a correlation between BRAFV600E mutation and CNS-risk site involvement, consistent with the results of a previous study.12 BRAFV600E gene mutation was not associated with RO+, multisystem involvement, initial 6-week treatment response, or disease progression / reactivation in this study, consistent with previous research reports.11,14,25 A recent study by Tandon et al.15 reported no significant correlation between BRAFV600E expression and clinical outcomes (early treatment response, reactivation rate, and late sequelae) in children with LCH, which is consistent with the findings of this study. A previous study14 reported a significant correlation between BRAFV600E gene mutation and increased PD-L1 expression; however, this association was not observed in this study. Additional investigation is required to confirm the correlation between BRAFV600E and PD-L1. Therefore, BRAFV600E gene mutation may not be used as a biomarker for risk stratification in LCH currently.
The effect of adding dabrafenib on the induction response and outcomes during follow-up requires confirmation through a clinical trial. In this study, only 12 cases (due to the relatively small sample size) were BRAFV600E mutation-positive, and 2 of these cases did not receive dabrafenib treatment. Consequently, our study did not perform statistical analysis or evaluation of the therapeutic effect of dabrafenib.
The expression of PD-L1 in CD11C cells within lung lesions of LCH mice was significantly higher.26 A study22 conducted in Japan found that the expression rates of programmed cell death-1 and PD-L1 in six patients with musculoskeletal LCH were 16.6% and 83.3%, respectively, suggesting that the programmed cell death-1 / PD-L1 immune checkpoint molecules may play a role in the microenvironment of musculoskeletal LCH. Another study14 with a large sample size reported that the positive expression rate of PD-L1 in LCH was 32%, showing no association with age, gender, multisystem involvement, or RO+. This is consistent with the findings of our study, where the PD-L1 expression rate was 35.5%. This study showed that PD-L1 expression was not correlated with the initial 6-week treatment response and progression/reactivation of LCH, consistent with recent findings by Tandon et al.15 where they found that PD-L1 was not significantly correlated with the clinical outcomes (early treatment response, reactivation rate, and late sequelae) of children with LCH. However, Zeng et al.14 reported that BRAF mutation and increased PD-L1 expression were independent predictors of adverse disease-free survival, and BRAFV600E mutation was significantly correlated with increased PD-L1 expression. This study found a correlation between PD-L1 expression and skin involvement (however, there were only three cases of children with skin involvement) but not with BRAFV600E mutation, bone, lymph node, CNS or CNS-risk site involvement. Therefore, the correlation between PD-L1 and LCH requires further research, and currently, it may not be used as a biomarker for risk stratification in LCH.
CTSS is primarily found in the lysosomal/endosomal compartment of antigen-presenting cells (such as B cells, macrophages, and dendritic cells), but it can also be produced by epithelial cells, smooth muscle cells, endothelial cells, and neutrophils.19 Moreover, CTSS can also originate from tumor cells themselves or from other cell types in the tumor microenvironment, such as endothelial cells, macrophages, and T cells.16 The pathological feature of LCH is granulomatous lesions, consisting of CD1a+ and CD207+ histiocytes/dendritic cells and abundant inflammatory background cells, including T cells, neutrophils, eosinophils, B cells, monocytes, macrophages, and multinucleated giant cells1, while the predominant cellular populations in the immune microenvironment are M2-polarized macrophages and regulatory T cells.27 Therefore, immunohistochemical detection in this study showed that CTSS was expressed in all 31 LCH patients, and it was associated with age ≤ 2 years. CTSS from infiltrating immune cells and endothelial cells in the tumor further influences the tumor microenvironment16, and studies have confirmed that CTSS mutations induce a tumor-promoting immune microenvironment in follicular lymphoma.28 Thus, considering the expression of CTSS in LCH lesion tissues, we speculate that CTSS may be involved in the pathogenesis of LCH.
Given the expression of CTSS in LCH lesion tissues, CTSS may potentially serve as a therapeutic target. The reasons are as follows: Firstly, loss of CTSS activity reduces lymphoma growth by limiting communication with CD4+ T follicular helper cells, while inducing antigen diversification and activation of CD8+ T cells.29 Secondly, CTSS inhibition has non-redundant therapeutic potential in enhancing anti-tumor immune responses in indolent and invasive lymphomas.29 Finally, targeting CTSS can induce autophagy by activating the epidermal growth factor receptor (EGFR) / rat sarcoma virus oncogene (Ras) / mitogen-activated protein kinase (MEK) / extracellular signal-regulated kinase (ERK) signaling pathway30, indicating that it is a potential therapeutic target.17
The limitations of this study mainly include the relatively small sample size (associated with the rarity of the disease) and the possibility of admission rate bias. Future research should focus on multi-center studies with larger sample sizes or the construction of LCH cell lines/animal models to further investigate the role of the CTSS gene in the pathogenesis of LCH.
In summary, CTSS is expressed positively in LCH, and its expression level is associated with age ≤ 2 years. BRAFV600E gene mutation, PD-L1, and CTSS may not be associated with the prognosis of LCH.
Acknowledgements
We would like to thank our colleagues for clinical management.
Ethical approval
The study was approved by Medical Ethics Committee of the Affiliated Hospital of Qingdao University (date: 19.06.2023, number: QYFY WZLL 27894). Informed consent was obtained from their guardians.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
| Table V. Characteristics of children aged ≤ 2 years (n=35). | |||
|---|---|---|---|
| Characteristics |
|
|
|
|
CNS, central nervous system; LCH, Langerhans cell histiocytosis. * Fisher’s exact test. |
|||
| Multisystem involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| Risk organ involvement |
|
||
| Yes |
|
|
|
| No |
|
|
|
| BRAFV600E gene mutation |
|
||
| Yes |
|
|
|
| No |
|
|
|
References
- Gao XM, Li J, Cao XX. Signaling pathways, microenvironment, and targeted treatments in Langerhans cell histiocytosis. Cell Commun Signal 2022; 20: 195. https://doi.org/10.1186/s12964-022-00917-0
- Sconocchia T, Foßelteder J, Sconocchia G, Reinisch A. Langerhans cell histiocytosis: current advances in molecular pathogenesis. Front Immunol 2023; 14: 1275085. https://doi.org/10.3389/fimmu.2023.1275085
- Lang M, Cai HC, Lin H, et al. Clinical features, genomic profiling, and outcomes of adult patients with unifocal Langerhans cell histiocytosis. Orphanet J Rare Dis 2023; 18: 372. https://doi.org/10.1186/s13023-023-02989-8
- Tantawy AA, Ragab IA, Elsherif NH, Makkeyah SM, AbdelRaheem HG, Elsantiel HI. Egyptian experience in Langerhans cells histiocytosis: frequent multisystem affection and reactivation rates. Pediatr Hematol Oncol 2020; 37: 696-706. https://doi.org/10.1080/08880018.2020.1790703
- Tang JJ, Xu XJ, Wang YC, et al. Clinical manifestations of Langerhans cell histiocytosis with multisystem involvement in 53 children. Zhonghua Er Ke Za Zhi 2021; 59: 37-41. https://doi.org/10.3760/cma.j.cn112140-20200605-00584
- Cui L, Wang CJ, Lian HY, et al. Clinical outcomes and prognostic risk factors of Langerhans cell histiocytosis in children: results from the BCH-LCH 2014 protocol study. Am J Hematol 2023; 98: 598-607. https://doi.org/10.1002/ajh.26829
- Bielamowicz K, Dimitrion P, Abla O, et al. Langerhans cell histiocytosis: NACHO update on progress, chaos, and opportunity on the path to rational cures. Cancer 2024; 130: 2416-2439. https://doi.org/10.1002/cncr.35301
- Hayase T, Saito S, Shioda Y, et al. Analysis of the BRAF and MAP2K1 mutations in patients with Langerhans cell histiocytosis in Japan. Int J Hematol 2020; 112: 560-567. https://doi.org/10.1007/s12185-020-02940-8
- Ozer E, Sevinc A, Ince D, Yuzuguldu R, Olgun N. BRAF V600E Mutation: a significant biomarker for prediction of disease relapse in pediatric Langerhans cell histiocytosis. Pediatr Dev Pathol 2019; 22: 449-455. https://doi.org/10.1177/1093526619847859
- Tang X, Gao J, Ma ZG, et al. Clinical and prognostic characteristics of 95 cases of Langerhans cell histiocytosis in children: a single-institute experience from 2013 to 2020. Ann Med 2021; 53: 1537-1546. https://doi.org/10.1080/07853890.2021.1966085
- Feng S, Han L, Yue M, et al. Frequency detection of BRAF V600E mutation in a cohort of pediatric Langerhans cell histiocytosis patients by next-generation sequencing. Orphanet J Rare Dis 2021; 16: 272. https://doi.org/10.1186/s13023-021-01912-3
- Kemps PG, Zondag TCE, Arnardóttir HB, et al. Clinicogenomic associations in childhood Langerhans cell histiocytosis: an international cohort study. Blood Adv 2023; 7: 664-679. https://doi.org/10.1182/bloodadvances.2022007947
- Cournoyer E, Ferrell J, Sharp S, et al. Dabrafenib and trametinib in Langerhans cell histiocytosis and other histiocytic disorders. Haematologica 2024; 109: 1137-1148. https://doi.org/10.3324/haematol.2023.283295
- Zeng K, Wang Z, Ohshima K, et al. BRAF V600E mutation correlates with suppressive tumor immune microenvironment and reduced disease-free survival in Langerhans cell histiocytosis. Oncoimmunology 2016; 5: e1185582. https://doi.org/10.1080/2162402X.2016.1185582
- Tandon S, Weitzman S, Joyce B, et al. Expression and clinical correlation of PD-1/PD-L1 and VE1(BRAFp.V600E) in pediatric Langerhans cell histiocytosis. Mediterr J Hematol Infect Dis 2023; 15: e2023035. https://doi.org/10.4084/MJHID.2023.035
- McDowell SH, Gallaher SA, Burden RE, Scott CJ. Leading the invasion: the role of cathepsin S in the tumour microenvironment. Biochim Biophys Acta Mol Cell Res 2020; 1867: 118781. https://doi.org/10.1016/j.bbamcr.2020.118781
- Smyth P, Sasiwachirangkul J, Williams R, Scott CJ. Cathepsin S (CTSS) activity in health and disease - a treasure trove of untapped clinical potential. Mol Aspects Med 2022; 88: 101106. https://doi.org/10.1016/j.mam.2022.101106
- Tao P, Xu P. Research progress in regulatory role of cathepsin S in development of hepatocellular carcinoma. Mil Med Sci 2020; 44: 634-638. https//doi.org/10.7644/j.issn.1674-9960.2020.08.014
- Brown R, Nath S, Lora A, et al. Cathepsin S: investigating an old player in lung disease pathogenesis, comorbidities, and potential therapeutics. Respir Res 2020; 21: 111. https://doi.org/10.1186/s12931-020-01381-5
- Zhang L, Chen S. Research progress of Cathepsin S in respiratory diseases. J Med Theor & Prac 2022; 35: 208-210. https//doi.org/10.19381/j.issn.1001-7585.2022.02.009.
- Malicherova B, Burjanivova T, Grendar M, et al. Droplet digital PCR for detection of BRAF V600E mutation in formalin-fixed, paraffin-embedded melanoma tissues: a comparison with Cobas® 4800, Sanger sequencing, and allele-specific PCR. Am J Transl Res 2018; 10: 3773-3781.
- Hashimoto K, Nishimura S, Sakata N, Inoue M, Sawada A, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in the pathogenesis of musculoskeletal Langerhans cell histiocytosis: a retrospective study. Medicine (Baltimore) 2021; 100: e27650. https://doi.org/10.1097/MD.0000000000027650
- Azim HA, Peccatori FA, Brohée S, et al. RANK-ligand (RANKL) expression in young breast cancer patients and during pregnancy. Breast Cancer Res 2015; 17: 24. https://doi.org/10.1186/s13058-015-0538-7
- Luo L, Zhang A, Wang P, et al. Clinical features and curative effect analysis of 92 children with Langerhans cell histiocytosis. J China Pediatr Blood Cancer 2022; 27: 173-183. https//doi.org/10.3969/j.issn.1673-5323.2022.03.006
- Feng C, Li Y, Peng X, Xiong X, Weng W, Wu P. A retrospective analysis on Langerhans cell histiocytosis and the association between BRAF V600E mutation status and clinical features in children. Chin J Appl Clin Pediatr 2021; 36: 848-852. https//doi.org/10.3760/cma.j.cn101070-20200119-00081
- Sengal A, Velazquez J, Hahne M, et al. Overcoming T-cell exhaustion in LCH: PD-1 blockade and targeted MAPK inhibition are synergistic in a mouse model of LCH. Blood 2021; 137: 1777-1791. https://doi.org/10.1182/blood.2020005867
- Paredes SE, Almeida LY, Trevisan GL, et al. Immunohistochemical characterization of immune cell infiltration in paediatric and adult Langerhans cell histiocytosis. Scand J Immunol 2020; 92: e12950. https://doi.org/10.1111/sji.12950
- Bararia D, Hildebrand JA, Stolz S, et al. Cathepsin S alterations induce a tumor-promoting immune microenvironment in follicular lymphoma. Cell Rep 2020; 31: 107522. https://doi.org/10.1016/j.celrep.2020.107522
- Dheilly E, Battistello E, Katanayeva N, et al. Cathepsin S regulates antigen processing and T cell activity in non-hodgkin lymphoma. Cancer Cell 2020; 37: 674-689.e12. https://doi.org/10.1016/j.ccell.2020.03.016
- Chen KL, Chang WS, Cheung CH, et al. Targeting cathepsin S induces tumor cell autophagy via the EGFR-ERK signaling pathway. Cancer Lett 2012; 317: 89-98. https://doi.org/10.1016/j.canlet.2011.11.015
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















