Abstract
Introduction. Hyperandrogenism is a clinical condition in girls, resulting from excessive androgen production originating from the adrenal glands or ovaries. The measurement of androgen hormones plays an essential role in supporting the clinical diagnosis. These hormone levels can be assessed using immunoassay methods or liquid chromatography – tandem mass spectrometry (LC-MS/MS). Our study aimed to assess the efficacy of hormone measurement with both methods in girls clinically diagnosed with hyperandrogenism.
Methods. Girls presenting with hyperandrogenism were included in this cross-sectional retrospective study. The exclusion criteria included a diagnosis of precocious puberty, classical congenital adrenal hyperplasia (CAH), adrenocortical tumors, and the use of medications known to affect androgen levels. Hormones measured simultaneously by both methods were compared. Regression analysis was performed to adjust hormone levels for age and pubertal stage. Receiver operating characteristic (ROC) analysis was performed based on diagnosis, and androgen hormones with the highest specificity and sensitivity for diagnosis were identified.
Results. A total of 96 girls with hyperandrogenism were included in the study. 60 (62.5%) were diagnosed with premature adrenarche (PA), 31 (32.3%) with polycystic ovary syndrome (PCOS), and 5 (5.2%) with non-classical congenital adrenal hyperplasia (NCCAH). Dehydroepiandrosterone sulfate (DHEAS) measured by LC-MS/MS was significantly lower (p<0.001) but less concordant with clinical diagnosis than electrochemiluminescence immunoassay in PA cases. The androgen hormone with the highest area under the curve (AUC) value was androstenedione for PCOS (AUC: 0.792), and 17-hydroxyprogesterone (AUC: 0.994) using LC-MS/MS for NCCAH.
Conclusions. The measurement of DHEAS levels by both methods has low specificity. Androstenedione and total testosterone measured by LC-MS/MS had the highest sensitivity and specificity in PCOS.
Keywords: hyperandrogenism, liquid chromatography – tandem mass spectrometry, premature adrenarche, polycystic ovary syndrome
Introduction
Hyperandrogenism is a clinical condition caused by excessively high levels of androgen hormones, which result from increased ovarian, adrenal, or peripheral androgen production.1 The etiology of hyperandrogenism varies between the prepubertal and pubertal periods.
The primary cause of prepubertal hyperandrogenism is premature adrenarche (PA). In girls, PA typically presents with clinical signs before the age of 8 years, including early axillary and pubic hair growth, apocrine body odor, greasy hair, acne, and transient growth acceleration. Adrenarche is characterized by increased adrenal androgen hormone precursors, primarily dehydroepiandrosterone (DHEA) and its sulfate form (DHEAS). A serum DHEAS level exceeding 40 µg/dL is accepted as a biochemical marker of adrenarche. It is essential to exclude other pathological causes of androgen excess, such as non-classical congenital adrenal hyperplasia (NCCAH), precocious puberty, and androgen-producing tumors, and exogenous androgen exposure.2,3
After the onset of puberty, polycystic ovary syndrome (PCOS) emerges as the most common cause of hyperandrogenism. The primary symptoms of PCOS include menstrual irregularities and hyperandrogenism symptoms such as treatment-resistant acne and hirsutism.4 The international consensus on diagnostic criteria for PCOS involves identifying evidence of ovulatory dysfunction through abnormal menstrual patterns, along with clinical and biochemical hyperandrogenism. Clinicians should first evaluate total or free testosterone levels to assess biochemical hyperandrogenism. In cases with normal testosterone levels, it is recommended that DHEA and androstenedione (AS) be measured secondarily.5 However, it should be noted that biochemical hyperandrogenism is not essential for the diagnosis of PCOS mainly as there is no consistent correlation between clinical and biochemical hyperandrogenism.
NCCAH should be considered in the differential diagnosis of PA and PCOS. Heterogeneous CYP21A2 variants primarily cause a mild deficiency of the 21-hydroxylase enzyme.6 Clinicians may perform an adrenocorticotropic hormone (ACTH) stimulation test to rule out NCCAH. However, some clinicians suggest that basal serum 17-hydroxyprogesterone (17OHP) levels can be helpful for exclusion. Genetic analysis should be conducted for a conclusive diagnosis if an elevated 17OHP level is detected at baseline or after ACTH stimulation.7,8
Accurate measurement of androgen hormones and precursors is essential for differential diagnosis and clinical evaluation. Immunoassay methods, including enzyme-linked immunosorbent assay (ELISA) and electrochemiluminescence immunoassay (ECLIA) are commonly used for measuring steroid hormones due to their simplicity, cost-effectiveness, and sensitivity. However, because of the matrix effect and low specificity, immunoassay methods can be prone to inaccuracies when measuring steroid hormones.9,10 The liquid chromatography – tandem mass spectrometry (LC-MS/MS) method offers several advantages, such as effectively measuring low concentrations of steroid hormones, reducing interference from analytes, and simultaneously measuring multiple hormone levels.11
This study aimed to assess the efficacy of androgen hormone levels measured by both methods in girls presenting with clinical hyperandrogenism.
Materials and Methods
Study population
Prepubertal and pubertal girls from the pediatric population presenting with symptoms of hyperandrogenism at a single tertiary center between January 2017 and December 2018 were included in the study. Cases diagnosed with precocious puberty, classical CAH, or adrenocortical tumors, along with those undergoing medical therapy that could interfere with androgen hormone measurement, were excluded from the study. Demographic data, auxologic measurements, bone age assessments, laboratory results, and molecular variant analysis of the cases were obtained retrospectively from the clinical records. The diagnoses of the cases were determined by experienced pediatric endocrinologists based on the latest guidelines.12 Cases younger than 8 years with adrenarche symptoms onset, after other causes of hyperandrogenism were excluded, were diagnosed with premature pubarche. PCOS was diagnosed in patients whose menstrual irregularities persisted for two years after menarche and who had clinical hyperandrogenism. A standard dose ACTH test was performed on patients with baseline 17OHP levels >2 ng/mL. Genetic variant analysis was performed in patients with stimulated 17OHP levels >10 ng/mL, and cases with a detected biallelic variant were accepted as NCCAH.
This study was approved by Ege University Medical Research Ethics Committee (Approval No: 18.11T/27), and the principles of the Declaration of Helsinki were followed during this study.
Hormone measurements
The hormones simultaneously measured by immunoassay (IA) and LC-MS/MS methods were 17OHP, DHEAS, and total testosterone (TT). Hormone levels were assessed on the third day of menstruation in PCOS patients. Serum TT and DHEAS concentration were analyzed using the electrochemiluminescence (ECLIA) method (Cobas e-801, Roche Diagnostics GmbH, Germany), serum 17OHP levels were analyzed using the enzyme-linked immunosorbent assay (ELISA) method (Diamtera, SNL, Italy). Plasma concentrations of 17OHP, DHEA, DHEAS, TT, dihydrotestosterone (DHT), and AS were measured using LC-MS/MS (Eureka Lab Division, Code LC72310; 6460 Triple Quadrupole LC-MS/MS, Agilent Technologies, USA). The analytical procedure included protein precipitation using a reagent containing internal standards, followed by centrifugation, dilution, and direct injection into the LC-MS/MS system.
The instrument was operated in multiple reaction monitoring (MRM) mode under positive and negative atmospheric pressure chemical ionization (APCI). The analytical column used was an RRHD Eclipse Plus C18 column with dimensions 50 × 2.1 mm and a particle size of 1.8 µm. Fragmentation parameters for each analyte included the Q1 and Q3 mass-to-charge (m/z) ratios and compound-specific collision energies. The transitions monitored were m/z 331 to 109 for 17-OHP, 271.2 to 231.1 for DHEA, 289.1 to 97 for TT, and 273.1 to 255 for both DHT and AS, with collision energies ranging from 20 to 30 eV.
The analytical performance characteristics were as follows: lower limits of quantification (LLOQ) were 0.015 ng/mL for 17OHP, 0.1 ng/mL for DHEA, 0.6 ng/mL for DHEAS, 0.003 ng/mL for TT, 0.02 ng/mL for DHT, and 0.003 ng/mL for AS. The method also demonstrated lower limits of detection (LLOD) ranging from 0.001 to 0.2 ng/mL, depending on the analyte.
Precision of the assay was evaluated at low, medium, and high concentrations. Intra-day coefficients of variation (%CV) values ranged from 2.0% to 15.2%, and inter-day %CV values ranged from 2.2% to 15.9%, with the highest variability observed at the lowest concentrations of DHEA and TT. The recovery rate for all analytes was reported to be approximately 100% based on the manufacturer’s validation data.
Genetic variant analysis
Genetic analysis of the CYP21A2 gene, which encodes the 21-hydroxylase enzyme, was conducted on all cases showing an increase in the standard dose ACTH stimulation test. Variant analysis of the CYP21A2 gene was performed using a reverse dot blot (RDB) assay. Using the RDB assay, an assessment was conducted on the eleven regions of the CYP21A2 gene where variants are most commonly detected. The results were confirmed by performing complete gene sequencing on cases where the RDB assay was positive and those with clinical suspicion despite negative results.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 25.0 (SPSS Inc, Chicago, IL, USA). A p-value less than 0.05 was used to indicate statistical significance. The Kolmogorov-Smirnov test was used to assess normality. Descriptive statistics for parametric data were expressed as mean and standard deviation values. Non-parametric data of hormone levels were expressed as median and interquartile range. The comparison of androgen hormones TT, DHEAS, and 17OHP, measured simultaneously by LC-MS/MS and IA methods, was performed using the Wilcoxon signed-rank test. Given the effect of age and Tanner stage on androgen hormone levels, regression analysis was performed to obtain adjusted hormone levels. Receiver operating characteristic (ROC) curve analysis was performed in clinically diagnosed cases, and the assay with the highest area under the curve (AUC) was identified. The optimal cut-off value was determined using the Youden index by identifying the point with the highest sensitivity and specificity.
Results
This study included 96 girls with hyperandrogenism. The diagnostic distribution was as follows: 60 (62.5%) had PA, 31 (32.3%) had PCOS, and 5 (5.2%) had NCCAH. One NCCAH case was pubertal, while the others were prepubertal. The hormone levels obtained using the LC-MS/MS and immunoassay methods during the diagnostic evaluation are shown in Table I.
|
Data presented as median (Q1-Q3). 17OHP: 17-hydroxyprogesterone, DHEAS: Dehydroepiandrosterone sulfate, ECLIA: Electrochemiluminescence, ELISA: Enzyme-linked immunosorbent assay, LC-MS/MS: Liquid chromatography – tandem mass spectrometry, NCCAH: Non-classical congenital adrenal hyperplasia, PA: Premature adrenarche, PCOS: Polycystic ovary syndrome, TT: Total testosterone. |
||||
| Table I. Comparison of androgen hormone levels measured simultaneously by immunoassay and LC-MS/MS according to diagnostic subgroups. | ||||
| Analyte | Method |
|
|
|
| 17OHP (ng/dL) | LC-MS/MS |
|
|
|
| ELISA |
|
|
|
|
| p value |
|
|
|
|
| DHEAS (µg/dL) | LC-MS/MS |
|
|
|
| ECLIA |
|
|
|
|
| p value |
|
|
|
|
| TT (ng/dL) | LC-MS/MS |
|
|
|
| ECLIA |
|
|
|
|
| p value |
|
|
|
|
The mean age of the PA cases was 6.7±0.9 years, their body weight standard deviation score (SDS) was 1.10±1.04, height SDS was 0.92±1.2 and BMI SDS was 0.93±0.91. The bone and chronological age difference in PA cases was 1.4±1.29 years. The comparison of hormone measurements by the two methods revealed that hormone levels of DHEAS measured by the ECLIA method were significantly higher (p<0.001). The scatter dot plot showed that 11% of cases had DHEAS levels below the biochemical adrenarche threshold value by both methods (Fig. 1). It was also noted that 39% of the cases had DHEAS levels above the threshold according to the ECLIA but below the threshold when measured by LC-MS/MS. However, no cases were found where DHEAS levels were measured above the threshold by LC-MS/MS but below the threshold by ECLIA. ROC analysis of adjusted hormone levels showed that the highest AUC value was observed for DHEAS measured by the ECLIA method (AUC: 0.728). A cut-off value was 44 µg/dL with a sensitivity of 84% and specificity of 62% (Fig. 2A).
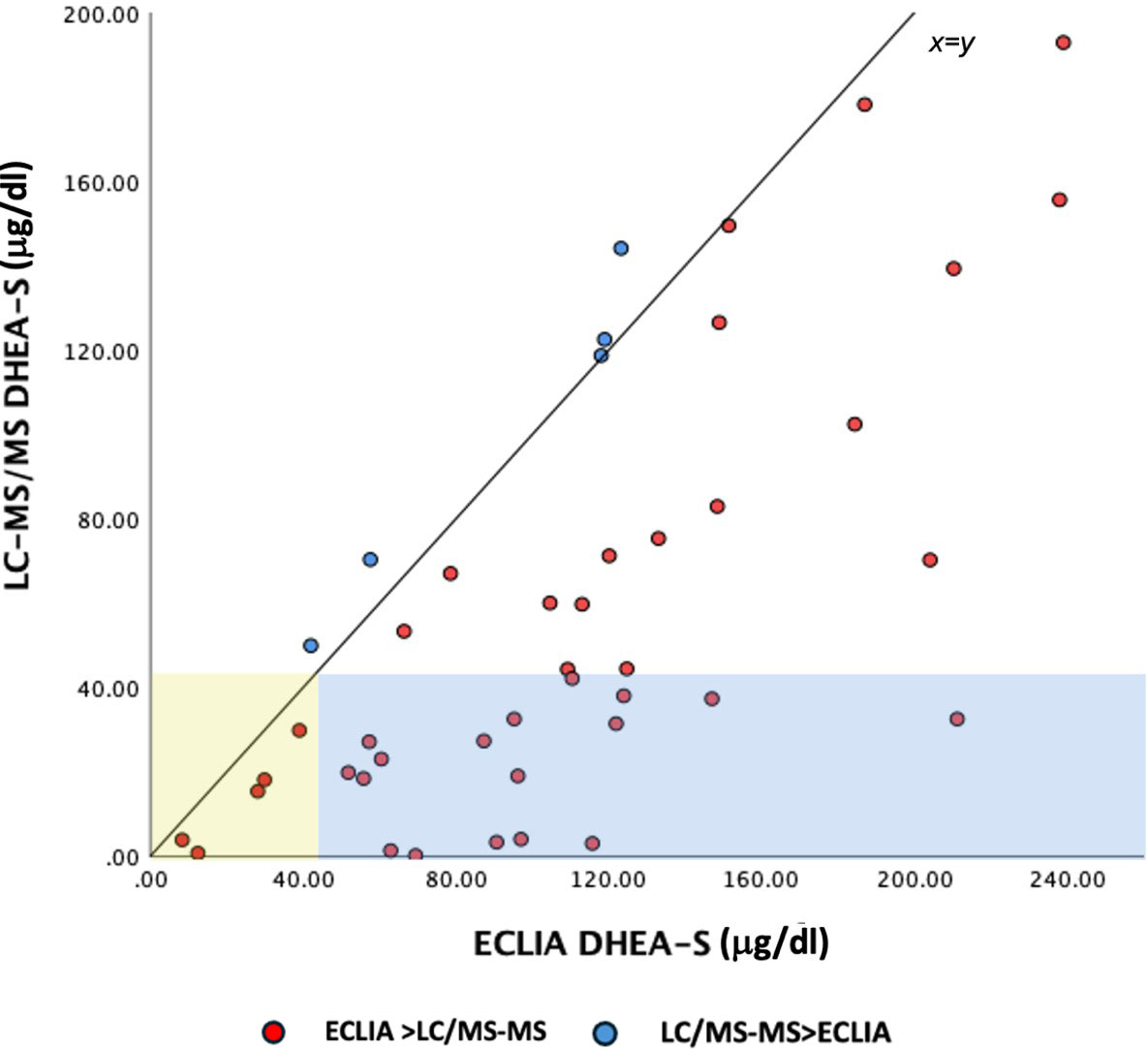
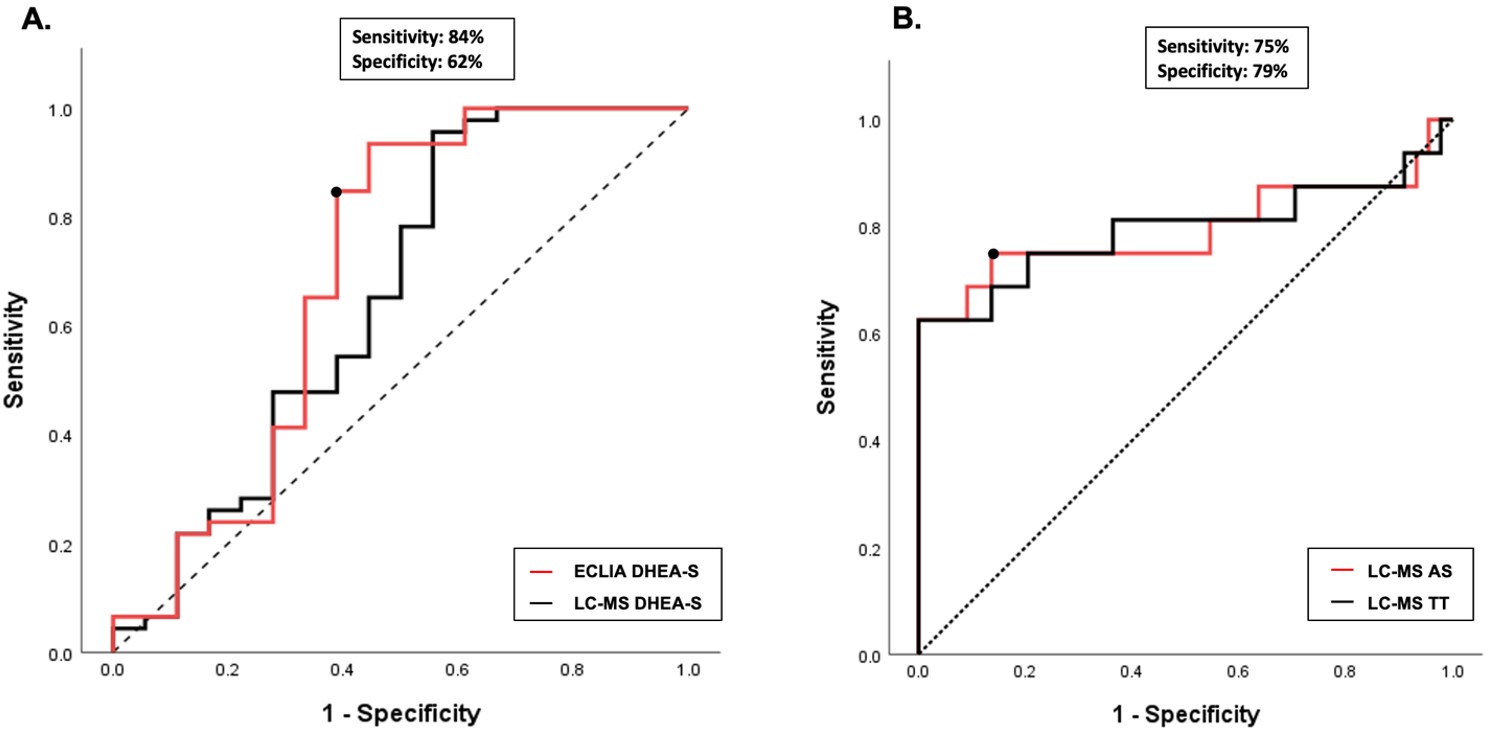
The mean age of PCOS cases was 15.3±1.2 years, and their body weight SDS was 0.95±1.55, height SDS was -0.1±1.2 and BMI SDS was 1.03±1.30. No significant difference was found in the comparison of TT levels performed simultaneously by the ECLIA and LC-MS/MS methods (p=0.677). ROC analysis showed that TT and AS levels measured with the LC-MS/MS method had the highest AUC values in diagnosis of PCOS (AUC: 0.792). AS level measured by LC-MS/MS with cut-off values of 49 µg/dL yielded a sensitivity of 79% and specificity of 82%, while TT cut-off of 36 ng/dL resulted in a sensitivity of 83% and specificity 68% (Fig. 2B).
Genetic variant analysis was performed on 41 cases. Variants were detected in 5 cases, while variant analysis was negative in 36 cases. Among these variant negative cases, 18 (50%) of them were diagnosed with PA, and 18 (50%) of them had PCOS. The V281L variant of CYP21A2 was detected in 4 cases, and the P453S variant in 1 case. The mean age of NCCAH cases was 8.2±2.78 years, their height SDS was 0.72±1.5, their body weight SDS was 1.76±1.1, and their BMI SDS was 1.65±0.84. Among the five cases, four presented with premature adrenarche and were at Tanner stage 2, while one case was diagnosed during the evaluation of menstrual irregularities with Tanner stage 5. No statistically significant difference was found between the 17OHP levels measured by LC-MS/MS and ELISA methods (p=0.144).
Discussion
The auxiliary data and hormonal evaluation of girls diagnosed with PA, PCOS, and NCCAH are presented here. The mean height, weight, and BMI SDS were above the average for healthy children of the same age in all diagnostic subgroups but still within normal limits. Current studies show that PA, PCOS, and NCCAH cases tend to have higher height, weight, and BMI SDS.13-15
In the present study, DHEAS levels, the biochemical indicator of adrenarche, measured by the LC-MS/MS method, were significantly lower than those of ECLIA.10 The percentage of cases with DHEAS levels below the threshold by both methods was lower than that reported in the literature.15
Biochemical adrenarche is defined as DHEAS levels exceeding 40 µg/dl. However, a definitive threshold has not been established for PA. Measurement of DHEAS by LC-MS/MS is more sensitive compared to IA methods. In our study, although the two methods demonstrated comparable diagnostic performance, DHEAS measured by the ECLIA method exhibited higher sensitivity and specificity. Nonetheless, despite relatively high sensitivity, specificity was low. This is thought to be primarily due to the fact that DHEAS is not a bioactive androgen and is not strongly correlated with clinical findings.16 Recent studies have shown that 11-ketotestosterone (11-KT), a potent androgen receptor agonist, may serve as a more effective marker in identifying premature adrenarche.17 To the best of our knowledge, this is the first study in the literature to evaluate the efficacy of DHEAS in clinically diagnosed premature adrenarche cases.18 The International Evidence-based Guideline for Assessment and Management of Polycystic Ovary Syndrome recommends that total or free testosterone measurements be evaluated initially for biochemical hyperandrogenism in the diagnosis of PCOS. DHEAS and AS measurements can be analyzed secondarily in cases with normal testosterone levels.5 No significant difference was observed between TT measurements conducted by different methods. In the literature, the LC-MS/MS method is superior in evaluating low total testosterone levels. However, similar superiority has not been demonstrated in patients with hyperandrogenism.19
In our study, AS and TT levels measured by LC-MS/MS were found to have equal AUC values. However, AS demonstrated slightly higher specificity compared to TT at a similar level of sensitivity. The AUC values of AS and TT in our study are consistent with those reported in a recently published meta-analysis. However, in contrast to the meta-analysis, our findings demonstrated that AS exhibited similar sensitivity compared to TT.20
Due to the small number of cases, the evaluation of NCCAH cases is limited. However, similar to the literature, the effectiveness of 17OHP measurement with LC-MS/MS in diagnosing NCCAH cases with detected genetic variants has been demonstrated. In the present study, the most common CYP21A2 variant was V281L, which is consistent with the literature.6
Study limitations
The study’s main limitations are its retrospective design and the lack of a control group. Another area for improvement is the need for hormone values from alternative steroidogenesis pathways. Additionally, due to the small number of detected genetic variants in NCCAH cases, the evaluation should be considered suboptimal. Prospective studies are needed to evaluate the clinical efficacy of hormone measurement using the LC-MS/MS method.
Conclusion
The specificity of DHEAS measured by the ECLIA and LC-MS/MS is low in PA cases. Recent studies have reported 11-KT levels measured by LC-MS/MS as more useful in the diagnosis of PA. The sensitivity and specificity of AS and TT levels measured by LC-MS/MS in PCOS were similar to those in the literature. In conclusion, a detailed evaluation of the main and backdoor pathways of steroidogenesis in patients presenting with androgen excess is important for differential diagnosis.
Ethical approval
The study was approved by Ege University Medical Research Ethical Committee (date: November 16th, 2018, number: 18.11T/27). Before the study, an informed written consent form was obtained from the legal caregivers of all participants, and the principles of the Declaration of Helsinki performed this study.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Rosenfield RL. The search for the causes of common hyperandrogenism, 1965 to circa 2015. Endocr Rev 2024; 45: 553-592. https://doi.org/10.1210/endrev/bnae007
- Novello L, Speiser PW. Premature adrenarche. Pediatr Ann 2018; 47: e7-e11. https://doi.org/10.3928/19382359-20171214-04
- Rosenfield RL. Normal and premature adrenarche. Endocr Rev 2021; 42: 783-814. https://doi.org/10.1210/endrev/bnab009
- Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol 2020; 33: 445-447. https://doi.org/10.1016/j.jpag.2020.06.017
- Teede HJ, Tay CT, Laven JJE, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab 2023; 108: 2447-2469. https://doi.org/10.1210/clinem/dgad463
- Kurtoğlu S, Hatipoğlu N. Non-classical congenital adrenal hyperplasia in childhood. J Clin Res Pediatr Endocrinol 2017; 9: 1-7. https://doi.org/10.4274/jcrpe.3378
- Auer MK, Nordenström A, Lajic S, Reisch N. Congenital adrenal hyperplasia. Lancet 2023; 401: 227-244. https://doi.org/10.1016/S0140-6736(22)01330-7
- Ambroziak U, Kępczyńska-Nyk A, Kuryłowicz A, et al. The diagnosis of nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency, based on serum basal or post-ACTH stimulation 17-hydroxyprogesterone, can lead to false-positive diagnosis. Clin Endocrinol (Oxf) 2016; 84: 23-29. https://doi.org/10.1111/cen.12935
- Koal T, Schmiederer D, Pham-Tuan H, Röhring C, Rauh M. Standardized LC-MS/MS based steroid hormone profile-analysis. J Steroid Biochem Mol Biol 2012; 129: 129-138. https://doi.org/10.1016/j.jsbmb.2011.12.001
- Fanelli F, Gambineri A, Mezzullo M, et al. Revisiting hyper- and hypo-androgenism by tandem mass spectrometry. Rev Endocr Metab Disord 2013; 14: 185-205. https://doi.org/10.1007/s11154-013-9243-y
- Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol 2015; 173: D1-12. https://doi.org/10.1530/EJE-15-0338
- Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2018; 103: 4043-4088. https://doi.org/10.1210/jc.2018-01865
- Voutilainen R, Jääskeläinen J. Premature adrenarche: etiology, clinical findings, and consequences. J Steroid Biochem Mol Biol 2015; 145: 226-236. https://doi.org/10.1016/j.jsbmb.2014.06.004
- Ibáñez L, de Zegher F. Adolescent PCOS: a postpubertal central obesity syndrome. Trends Mol Med 2023; 29: 354-363. https://doi.org/10.1016/j.molmed.2023.02.006
- Barbot M, Mazzeo P, Lazzara M, Ceccato F, Scaroni C. Metabolic syndrome and cardiovascular morbidity in patients with congenital adrenal hyperplasia. Front Endocrinol (Lausanne) 2022; 13: 934675. https://doi.org/10.3389/fendo.2022.934675
- Augsburger P, Liimatta J, Flück CE. Update on adrenarche-still a mystery. J Clin Endocrinol Metab 2024; 109: 1403-1422. https://doi.org/10.1210/clinem/dgae008
- Rege J, Turcu AF, Kasa-Vubu JZ, et al. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab 2018; 103: 4589-4598. https://doi.org/10.1210/jc.2018-00736
- Liimatta J, du Toit T, Voegel CD, Jääskeläinen J, Lakka TA, Flück CE. Multiple androgen pathways contribute to the steroid signature of adrenarche. Mol Cell Endocrinol 2024; 592: 112293. https://doi.org/10.1016/j.mce.2024.112293
- Janse F, Eijkemans MJC, Goverde AJ, et al. Assessment of androgen concentration in women: liquid chromatography-tandem mass spectrometry and extraction RIA show comparable results. Eur J Endocrinol 2011; 165: 925-933. https://doi.org/10.1530/EJE-11-0482
- Bizuneh AD, Joham AE, Teede H, et al. Evaluating the diagnostic accuracy of androgen measurement in polycystic ovary syndrome: a systematic review and diagnostic meta-analysis to inform evidence-based guidelines. Hum Reprod Update 2025; 31: 48-63. https://doi.org/10.1093/humupd/dmae028
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















