Graphical Abstract
Abstract
Background. Adherence to antiretroviral therapy (ART) is a major challenge in pediatric human immunodeficiency virus (HIV) management, especially in young children due to medication formulation, administration difficulties, and psychosocial barriers. Single-tablet regimens (STRs) have been shown to improve adherence and viral suppression in adults and adolescents, yet their use in younger children remains limited. Bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) is an STR with a high genetic barrier to resistance, making it a promising option for pediatric patients with adherence difficulties.
Case Presentation. We report a case of a 2-year-old girl with perinatally acquired HIV who experienced persistent viremia despite multiple ART regimens. The mother received zidovudine prophylaxis during delivery, and the infant was started on zidovudine (AZT) prophylaxis on the first day of life. The patient’s ART history included AZT monotherapy at birth, followed by combination therapy with lamivudine (3TC), lopinavir/ritonavir (LPV/r), and later tenofovir/emtricitabine (TDF/FTC) with dolutegravir (DTG). Despite these regimens, poor adherence related to medication administration difficulties and caregiver challenges contributed to persistent viremia. A multidisciplinary team approach was implemented to address adherence barriers. Given the patient’s ongoing virological failure and resistance mutations (L76V and V179E), off-label use of BIC/FTC/TAF (50mg/200mg/25mg) was approved. The dosage was adjusted based on weight, and medication administration was closely monitored. Within one month of treatment, HIV RNA levels significantly declined from 1,800,000 to 207 copies/mL. Viral suppression was maintained over subsequent three-month intervals, with HIV RNA levels of 35, 40, and 43 copies/mL, alongside immune recovery as indicated by increased CD4 counts.
Conclusion. The successful off-label use of BIC/FTC/TAF in a treatment-refractory pediatric HIV case highlights its potential efficacy in young patients facing adherence challenges. Its high genetic barrier to resistance and favorable tolerability make it a promising option when standard therapies fail. Further research is needed to optimize pediatric ART strategies and expand access to STRs globally.
Keywords: pediatric HIV, antiretroviral therapy, adherence, single-tablet regimen, bictegravir/emtricitabine/tenofovir alafenamide, viral suppression
Introduction
Adherence to antiretroviral therapy (ART) is crucial for achieving and maintaining viral suppression in individuals living with human immunodeficiency virus (HIV). In pediatric populations, ensuring consistent adherence presents unique challenges due to factors such as medication formulation, palatability, dosing frequency, side effects, and the child’s developmental stage. Psychosocial and behavioral barriers, including family dynamics and caregiver involvement, further complicate adherence efforts. Studies have shown that adherence rates among children and adolescents can vary widely, with nonadherence rates ranging from 15% to 93%.1
The complexity of ART regimens, especially those requiring multiple tablets or doses per day, has been associated with lower adherence rates. Single-tablet regimens (STRs) have emerged as a strategy to simplify treatment, reduce pill burden, and improve adherence. In adult and adolescent populations, STRs have been linked to higher adherence rates, decreased hospitalizations, and a greater likelihood of achieving undetectable viral loads.2 However, the availability and approval of STRs for younger children have lagged, limiting their use in this vulnerable population.
Bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) is an STR that has demonstrated efficacy and safety in adults and adolescents. Recent studies have extended these findings to pediatric populations. For instance, a study involving children aged 2 years and older, weighing between 14 kg and 25 kg, showed that a low-dose co-formulated BIC/FTC/TAF regimen was well-tolerated and effective in maintaining viral suppression.3 The high genetic barrier to resistance offered by BIC/FTC/TAF makes it particularly advantageous in pediatric patients, who are at a higher risk for poor treatment adherence and subsequent virological failure.4
Despite these advancements, challenges remain in implementing STRs like BIC/FTC/TAF in younger children, particularly in regions where such formulations are not yet approved or available. This case report discusses the successful use of BIC/FTC/TAF in a 2-year-old child with HIV, highlighting the considerations and outcomes associated with off-label use to achieve viral suppression.
Case Presentation
A 2-year-old girl was referred to a tertiary healthcare center due to persistently high plasma viral load despite multiple ART regimens. She was born via cesarean section at 39 weeks, with a birth weight of 2960 grams. The mother’s HIV infection status was unknown until routine prenatal screening shortly before delivery, at which time she was found to be HIV-seropositive and had not received ART during pregnancy. The mother received zidovudine prophylaxis during delivery; however, the maternal HIV RNA viral load was positive after delivery, but the exact level at delivery was not available. Due to the limited availability of antiretroviral drugs at the initial healthcare center, the infant was started on zidovudine (AZT) prophylaxis on the first day of life until referral to a tertiary center within 48–72 hours postpartum. Upon admission to the tertiary center, triple ART was promptly initiated given the high-risk status of the mother-infant pair.
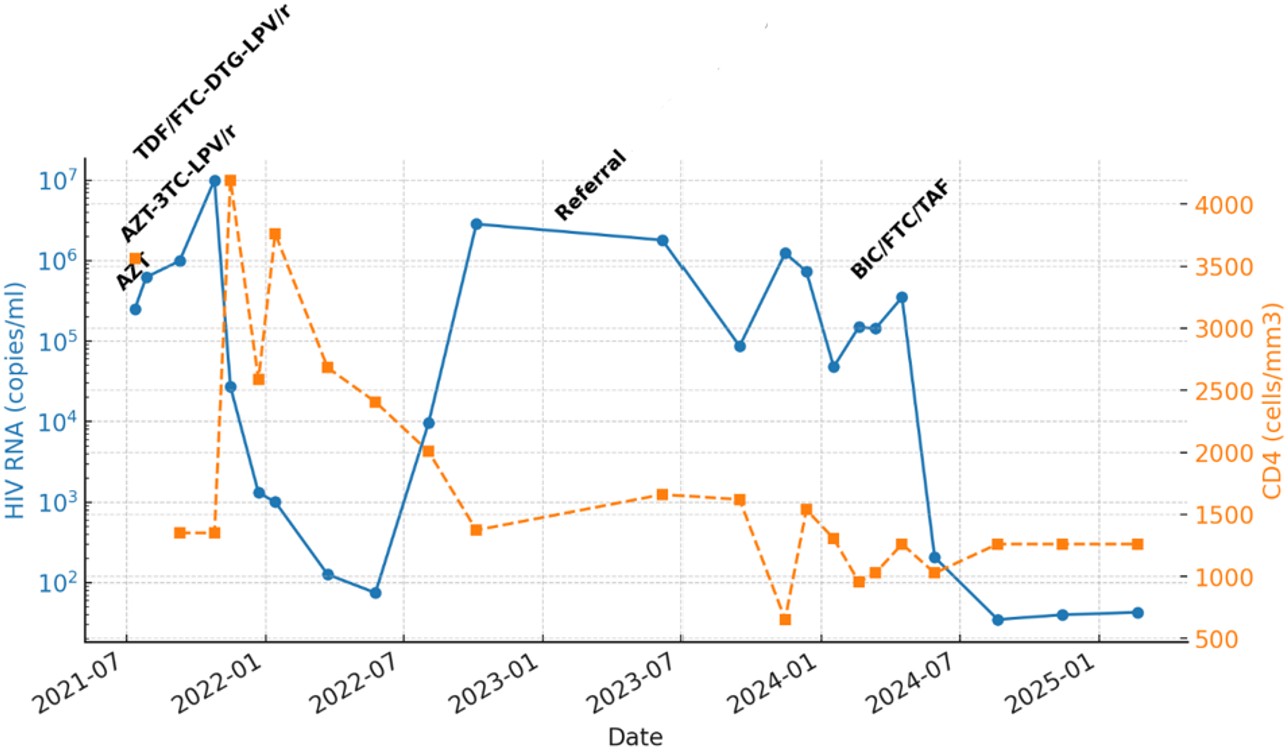
During the first month, triple ART consisting of AZT, lamivudine (3TC), and lopinavir/ritonavir (LPV/r) was initiated for the infant; however, the family reported intermittent difficulties in maintaining a consistent medication supply, which likely contributed to persistently high viral loads. The infant’s HIV RNA was 249,000 copies/mL and the CD4 count was 3561/mm³ during this period. Baseline genotypic resistance testing revealed no resistance mutations against protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors (NRTIs), or non-nucleoside reverse transcriptase inhibitors (NNRTIs). At two months of age, combination therapy with AZT, lamivudine (3TC), and lopinavir/ritonavir (LPV/r) was initiated. However, by the fourth month, viral suppression was not achieved (HIV RNA >10,000,000 copies/mL, CD4 count: 1351/mm³, stage 2 immunosuppression). Consequently, trimethoprim/sulfamethoxazole (TMP/SMX) and fluconazole prophylaxis were started, and the ART regimen was modified to tenofovir/emtricitabine (TDF/FTC), dolutegravir (DTG), and LPV/r.
Despite multiple ART adjustments, long-term viral suppression was not achieved. Reports from the previous treatment center indicated that the child was not consistently brought to follow-up visits, and medication adherence was poor. Family history revealed that the parents had ongoing conflicts, leading to the grandmother becoming the primary caregiver. The grandmother had limited knowledge about ART and HIV management, and she reported frequent difficulties in administering medications. Additionally, the child exhibited strong resistance to taking medications, frequently spitting them out or vomiting, particularly with the more complex multi-pill regimens.
At 24 months of age, the child was referred to a tertiary healthcare center for further evaluation and management. Upon admission, she was in good general condition with normal physical examination findings. Laboratory tests revealed HIV RNA of 1,800,000 copies/mL and a CD4 count of 1373/mm³. Additional evaluations included Quantiferon (IFN-ɣ) positivity, prompting further assessments with thoracic computed tomography (CT) and gastric aspirates. No evidence of Mycobacterium tuberculosis infection was found (negative acid-fast bacilli staining, polymerase chain reaction, and culture results). Isoniazid (INH) prophylaxis was initiated and completed over nine months.
The ART regimen of TDF/FTC, DTG, and LPV/r was continued, and a repeat resistance analysis was performed. Sequencing identified a newly acquired L76V mutation, conferring intermediate resistance to LPV/r, as well as the persistence of NNRTI resistance with V179E mutation. These findings suggested ongoing viral replication under suboptimal ART adherence, increasing the risk of developing further resistance.
Given the challenges in medication adherence, the pill burden and administration difficulties, and the risk of further resistance mutations, STR was considered. The child’s mother had achieved viral suppression on BIC/FTC/TAF (50mg/200mg/25mg), leading to the decision to explore this regimen for the child. However, the pediatric formulation (BIC/FTC/TAF 30mg/120mg/15mg) was not available in Turkey, necessitating off-label approval for the adult formulation. Approval was granted, and BIC/FTC/TAF (50mg/200mg/25mg) was administered in a dose adjusted to body weight (12.5 kg) by crushing one adult tablet in 5 mL of water and administering 3 mL once daily.
Within the first month of BIC/FTC/TAF initiation, HIV RNA levels significantly decreased to 207 copies/mL, indicating rapid virologic response. Subsequent viral load measurements at three-month intervals showed continued suppression, with values of 35, 40, and 43 copies/mL respectively, confirming sustained viral control. The CD4 count at the latest follow-up was 1261/mm³ (Fig. 1).
Additionally, structured counseling sessions were provided to the child’s caregivers to improve treatment adherence and understanding of ART importance. These included one-on-one sessions with a pediatric HIV specialist, a clinical psychologist, and a pharmacist to optimize medication administration strategies.
Written informed consent was obtained from the patient’s legal guardian for publication.
Discussion
This case highlights the importance of simplified regimens in pediatric HIV patients with adherence challenges and demonstrates the successful off-label use of BIC/FTC/TAF to achieve viral suppression in a previously treatment-refractory child. Pediatric HIV management presents significant challenges, particularly regarding medication adherence and the development of drug resistance. Younger children often struggle with adherence due to factors such as medication formulation, caregiver involvement, psychosocial barriers, and pill burden. This case highlights the importance of STRs) with high genetic barriers to resistance and the role of multidisciplinary approaches in improving adherence.
Pediatric administration of fixed-dose combination ARTs often requires formulation adjustments, such as tablet crushing or dissolving, to facilitate swallowing. Recent pharmacokinetic studies have highlighted the impact of these modifications on drug bioavailability. The SOLUBIC randomized crossover trial demonstrated that dissolving BIC/FTC/TAF tablets in water maintains comparable bioavailability to whole-tablet ingestion, whereas crushing the tablet significantly decreases exposure to emtricitabine and tenofovir alafenamide.5 Similarly, the HIV.gov pediatric treatment guidelines and recent reviews caution against tablet crushing due to potential reductions in drug absorption and therapeutic efficacy.6,7 Moreover, pediatric pharmacokinetic and safety data confirm the suitability of dissolved formulations for children aged 2 years and older, supporting the practice used in this case.8 These findings underscore the importance of careful consideration of formulation strategies to ensure effective viral suppression in pediatric HIV patients.
According to the European AIDS Clinical Society (EACS) guidelines, the recommended first-line ART regimen for children under two years of age typically consists of NRTIs (such as abacavir/lamivudine or zidovudine/lamivudine) in combination with a boosted PI (e.g., LPV/r) or an integrase inhibitor (INSTI) (e.g., raltegravir [RAL]).9 However, real-world applications often reveal suboptimal adherence to these regimens due to poor taste, high pill burden, and gastrointestinal side effects. The patient initially received AZT+3TC+LPV/r as per guidelines but failed to achieve viral suppression, requiring multiple regimen changes and ultimately necessitating an alternative, off-label approach.
Studies have demonstrated that children on multi-pill regimens have lower adherence rates compared to those on simplified regimens.1 A meta-analysis investigating ART adherence in pediatric populations found that nonadherence rates vary from 15% to 93%, with the most common barriers including caregiver-related difficulties, pill burden, and side effects.10 Simplified antiretroviral regimens like BIC/FTC/TAF have been associated with low discontinuation rates and high virologic suppression in both treatment-naive and treatment-experienced individuals, according to a systematic review and meta-analysis.11
A recent systematic review analyzing real-world outcomes of BIC/FTC/TAF therapy in diverse HIV populations found that treatment discontinuation rates were significantly lower in individuals on BIC/FTC/TAF compared to other ART regimens.11 This finding is particularly relevant for pediatric populations, as treatment retention is often compromised by medication side effects and regimen complexity. The study also emphasized that BIC/FTC/TAF use was associated with high rates of virologic suppression, reinforcing its potential role as an alternative therapy in children who struggle with adherence to traditional regimens. The patient’s case aligns with these findings, as the transition to BIC/FTC/TAF resulted in a rapid decline in viral load from 1,800,000 copies/ml to 207 copies/ml and sustained suppression over time.
Another phase 2/3 study investigating the pharmacokinetics and safety of co-formulated BIC/FTC/TAF in children aged 2 years and older reported that BIC/FTC/TAF maintained therapeutic drug concentrations, was well-tolerated, and achieved high virologic suppression rates.8 Notably, the study demonstrated that pediatric patients receiving BIC/FTC/TAF exhibited minimal adverse effects, which is crucial for maintaining adherence and treatment continuity. In our patient, despite previous poor adherence to multi-pill regimens, BIC/FTC/TAF administration resulted in significant clinical improvement, sustained virologic suppression, and an increase in CD4 count to 1261 cells/mm³. This case supports the potential for broader use of STRs in pediatric populations, particularly in those with complex adherence barriers.
Children with poor adherence are at increased risk of developing drug resistance, which can significantly limit future treatment options. BIC is particularly advantageous in such cases due to its high genetic barrier to resistance.4 Our patient developed the L76V mutation, conferring intermediate resistance to LPV/r, along with the NNRTI-associated V179E mutation. Despite these mutations, BIC/FTC/TAF achieved and maintained viral suppression, emphasizing the importance of high-barrier regimens in treatment-experienced children.
Multidisciplinary interventions play a crucial role in pediatric HIV management.12 A study on children living with HIV found that psychosocial support, caregiver education, and pharmaceutical counseling significantly improved ART adherence and virological outcomes.10 In our case, a multidisciplinary approach was implemented, including one-on-one caregiver education on ART adherence, structured counseling sessions with a psychologist to address the child’s medication refusal, and pharmacist-led medication administration training. These strategies helped stabilize adherence, leading to sustained viral suppression (final HIV RNA: 43 copies/mL) and improved immune response (final CD4 count: 1261 cells/mm³).
The off-label use of BIC/FTC/TAF in pediatric HIV remains an area of active investigation. Our case contributes to growing evidence that simplified regimens may serve as an effective alternative in children with adherence difficulties and drug resistance. However, further research is needed to assess the long-term safety and pharmacokinetics of BIC/FTC/TAF in younger children, its impact on adherence and clinical outcomes, and strategies to expand access to pediatric formulations in resource-limited settings.
Conclusion
This case underscores the importance of simplified ART regimens, multidisciplinary care, and individualized treatment strategies in pediatric HIV management. The successful off-label use of BIC/FTC/TAF in a treatment-refractory child highlights its potential efficacy in young patients with adherence challenges. The high genetic barrier to resistance and well-documented tolerability of BIC/FTC/TAF makes it a promising option, especially in cases where standard therapies fail due to nonadherence or drug resistance. Future research should focus on optimizing treatment strategies and expanding access to STRs for pediatric populations worldwide.
Acknowledgements
We would like to express our gratitude to Perinatal Virtual Clinic (PVC) (C Foster, N Mackie, H Lyall, A Bamford, A Bailey, R Weston and N Tickner) for their assistance in the management of this patient.
Ethical approval
Written informed consent was obtained from the patient’s legal guardian for publication.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Simoni JM, Montgomery A, Martin E, New M, Demas PA, Rana S. Adherence to antiretroviral therapy for pediatric HIV infection: a qualitative systematic review with recommendations for research and clinical management. Pediatrics 2007; 119: e1371-e1383. https://doi.org/10.1542/peds.2006-1232
- Sutton SS, Hardin JW, Bramley TJ, D’Souza AO, Bennett CL. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care 2016; 22: 242-248.
- Frange P, Veber F, Burgard M, Blanche S, Avettand-Fenoel V. Bictegravir/emtricitabine/tenofovir alafenamide in paediatrics: real-life experience from a French cohort (2019-2023). HIV Med 2024; 25: 299-305. https://doi.org/10.1111/hiv.13562
- Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019; 6: e364-e372. https://doi.org/10.1016/S2352-3018(19)30080-3
- Hocqueloux L, Lefeuvre S, Bois J, et al. Bioavailability of dissolved and crushed single tablets of bictegravir, emtricitabine, tenofovir alafenamide in healthy adults: the SOLUBIC randomized crossover study. J Antimicrob Chemother 2022; 78: 161-168. https://doi.org/10.1093/jac/dkac369
- Januszka JE, Drwiega EN, Badowski ME. Bictegravir/Emtricitabine/Tenofovir Alafenamide for HIV-1: what is the hidden potential of this emerging treatment? HIV AIDS (Auckl) 2023; 15: 705-711. https://doi.org/10.2147/HIV.S385877
- Chastain DB, Tu PJ, Brizzi M, et al. Managing modern antiretroviral therapy in the intensive care unit: overcoming challenges for critically ill people with human immunodeficiency virus. Open Forum Infect Dis 2024; 11: ofae213. https://doi.org/10.1093/ofid/ofae213
- Rodriguez CA, Natukunda E, Strehlau R, et al. Pharmacokinetics and safety of coformulated bictegravir, emtricitabine, and tenofovir alafenamide in children aged 2 years and older with virologically suppressed HIV: a phase 2/3, open-label, single-arm study. Lancet HIV 2024; 11: e300-e308. https://doi.org/10.1016/S2352-3018(23)00327-2
- Ambrosioni J, Levi L, Alagaratnam J, et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med 2023; 24: 1126-1136. https://doi.org/10.1111/hiv.13542
- Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS 2014; 28: 1945-1956. https://doi.org/10.1097/QAD.0000000000000316
- Chivite I, Berrocal L, de Lazzari E, et al. Effectiveness, safety and discontinuation rates of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) in people with HIV using real-world data: a systematic review and meta-analysis. J Antimicrob Chemother 2024; 79: 1775-1783. https://doi.org/10.1093/jac/dkae138
- Eley B, Nuttall J. Antiretroviral therapy for children: challenges and opportunities. Ann Trop Paediatr 2007; 27: 1-10. https://doi.org/10.1179/146532807X170448
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















