Abstract
Background. Non-Hodgkin lymphoma of the larynx in children is a rare condition. Diagnosis is difficult as its symptoms are usually attributed to respiratory tract infections and pubertal voice changes.
Case Presentations. We report two children diagnosed with laryngeal B-cell lymphoma based on imaging and histopathological findings. We also review other pediatric cases of laryngeal lymphoma documented in the literature, detailing tumor locations, lymphoma types, stages, etiological factors, and treatment regimens of these patients.
Conclusion. Diagnosis of laryngeal lymphoma is challenging. Although certain imaging features can be suggestive of the disease, a definitive diagnosis requires histopathological examination. Surgery is not required for the treatment, and chemotherapy is the main treatment approach. Early diagnosis is important.
Keywords: laryngeal lymphoma, non-Hodgkin lymphoma, children
Introduction
Primary lymphoma of the larynx accounts for less than 1% of laryngeal tumors and is extremely rare in childhood.1,2 The classic presenting symptoms of laryngeal tumors -dysphonia, dysphagia, dyspnea, and cervical lymphadenopathy- can often be mistaken for acute inflammatory diseases such as croup, acute epiglottitis, and retropharyngeal abscess.1,2 Diagnosis of primary laryngeal lymphoma is challenging because systemic symptoms are uncommon and symptoms typically remain localized for an extended period without progression.3 The primary treatment is chemotherapy.4 Early diagnosis is crucial as the prognosis is favorable in the early stages. Here, we present two pediatric cases of laryngeal lymphoma and provide a comprehensive review of previously reported cases of laryngeal lymphoma in children.
Case Presentations
Case 1
A 6-year-old male was admitted to the otolaryngology department with complaints of hoarseness, dyspnea, and wheezing for three months. There was no accompanying complaint of fever, weight loss, or night sweats. Firstly, a 10-day course of amoxicillin-clavulanic acid was administered by a local doctor. Since symptoms persisted for three months, magnetic resonance imaging (MRI) of the neck was performed. A 1.5x1.4 cm mass with markedly restricted diffusion was shown at the left aryepiglottic fold (Fig. 1). During direct laryngoscopy, a convex, regularly circumscribed mass was detected in the left arytenoid of the supraglottic area, and multiple biopsies were performed. Histopathological findings revealed discohesive medium/large-sized cells with eosinophilic cytoplasm. Immunohistochemistry staining showed LCA, CD20, CD19, CD43, CD30, MUM-1, and BCL-2 positivity. However, the staining with CD3, MPO, CD23, BCL-6, CD10, CD34, S100, synaptophysin, desmin, TdT, and pan-keratin were negative. A translocation involving c-MYC and IRF4/DUSP22 was not detected in neoplastic cells. Based on the immunohistochemical findings, the diagnosis of laryngeal mature B-cell non-Hodgkin lymphoma was made. Positron emission tomography - computed tomography (PET-CT) scan showed increased fluorodeoxyglucose (FDG) uptake only in the laryngeal mass, and low FDG uptake in bilateral cervical level 2 lymph nodes. There were no malignant cells in bone marrow and cerebrospinal fluid examinations. Serum biochemistry and LDH levels were within normal limits. He was classified as stage-II non-Hodgkin lymphoma and the FAB/LMB96 group B chemotherapy protocol was started. After completing treatment, he has been followed up without disease for the past two years.
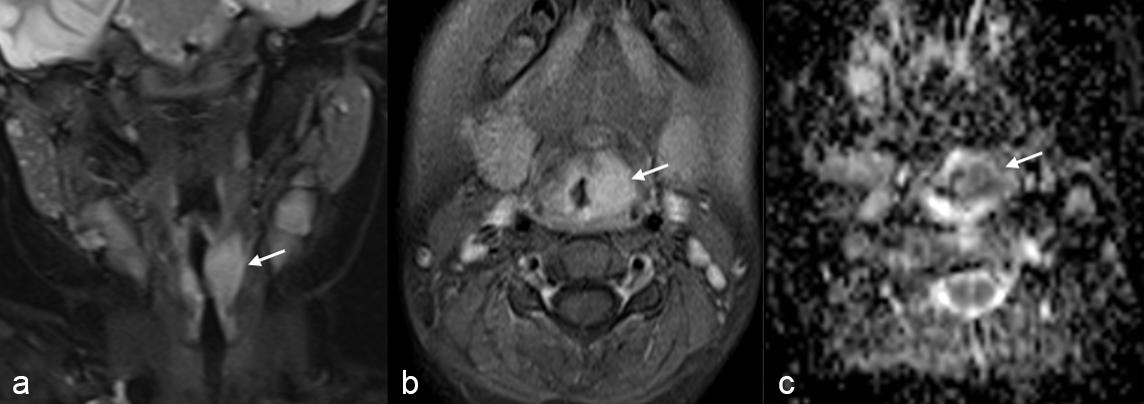
Case 2
A 15-year-old girl with serine/threonine kinase 4 (STK4) deficiency presented with progressively worsening hoarseness for one month. Initially seen in the immunology department and referred to the otolaryngology department, where she was prescribed oral gargle and clarithromycin. As she did not benefit from this treatment, a flexible laryngoscopy was performed, revealing a polypoid structure in the supraglottic area, filling the ventricular band and extending through the arytenoid to the aryepiglottic fold. Multiple punch biopsies were taken from the mass. Histopathological examination showed a neoplastic tissue having a wide necrotic area under the epithelium. Pleomorphic neoplastic cells had vesicular nucleus with multiple nucleoli and had high mitotic activity. Immunohistochemical staining for CD20, Bcl-2 and MUM1 were positive while CD3, CD10, BCL-6, PAX5, ALK, granzyme B, and Tia-1 staining were negative. Additionally, neoplastic cells were strongly positive in the Epstein-Barr encoding region (EBER) in situ hybridization test. She was diagnosed with Epstein-Barr virus (EBV)-positive diffuse large B cell lymphoma and referred to the pediatric oncology department. Although there was no peripheral lymphadenopathy at first admission, lymphadenopathy with a diameter of 1.5 cm in the left middle cervical chain and a diameter of 3x3 cm in the right axilla developed during the diagnostic investigation process. PET-CT scan showed increased FDG uptake in the posterior part of the left ventricular band and in the left cervical lymph nodes. Increased FDG uptake was also demonstrated in the nasopharynx, left palatine tonsil, bilateral axillary lymph nodes, mediastinal lymph nodes, and left pulmonary nodule (Fig. 2). Bone marrow biopsy and cerebrospinal fluid analysis were negative for tumor involvement. FAB/LMB96 group B chemotherapy protocol was started with the diagnosis of stage III B-cell lymphoma. At the end of 5 cycles of chemotherapy, a new lymph node was detected at the left supraclavicular region and excised. The histopathological examination confirmed the recurrence. Rituximab combined with chemotherapy including ifosfamide, carboplatin, and etoposide (ICE) was started as second-line treatment. Remission was achieved after 6 cycles of chemotherapy. Two months after remission, she underwent allogenic hematopoietic stem cell transplantation from an HLA-matched unrelated donor, for the treatment of her underlying immunodeficiency. She has been followed up without disease for four years.
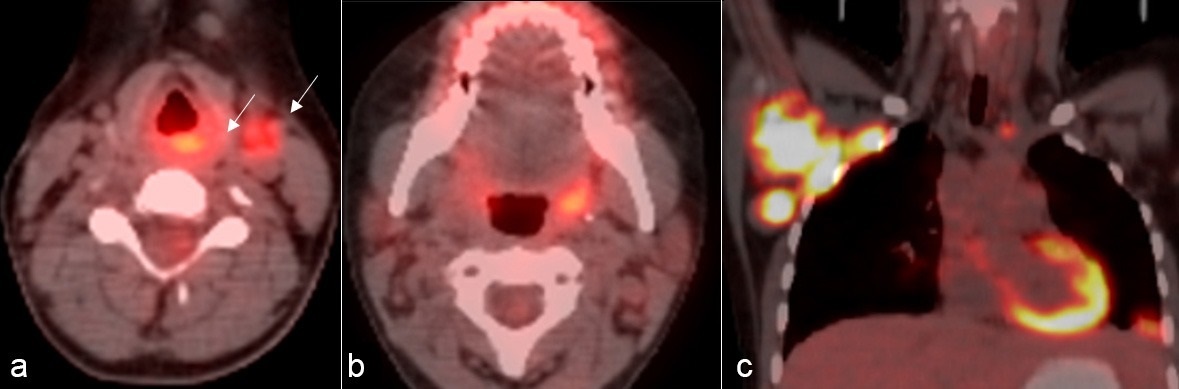
Discussion
Head and neck malignancies are rare in the pediatric age group and represent 5-12% of all pediatric cancers. The most common pediatric head and neck malignancies are lymphoma, rhabdomyosarcoma, thyroid carcinoma, nasopharyngeal carcinoma, and salivary gland malignancies.5,6 Arboleda et al. evaluated 367 head and neck tumors among the 7181 pediatric cancers diagnosed within 30 years. Lymphomas, carcinomas (nasopharynx, thyroid), and sarcomas (soft tissue and bone) constitute 52.8%, 22.9%, and 19.1% of them, respectively.6 No tumors of laryngeal origin, including lymphoma, were detected in this large pediatric head and neck tumors series.
Approximately 13% of pediatric lymphoma cases occur in the head and neck region and most commonly involve cervical lymph nodes. However, involvement of the extranodal regions such as Waldeyer’s ring, nasal cavity, paranasal sinuses, maxilla, and mandible are not unusual.1,5 Roh et al. reported that 40.5% of 37 head and neck lymphoma cases were located in extranodal sites and half of them were in Waldeyer’s ring.1 In a Brazilian series of 104 head and neck lymphoma patients, half of the cases were located in extranodal sites. There were no cases with larynx lymphoma in these series.6
Lymphoma of the larynx is extremely rare. A total of 200 cases have been recorded in the Surveillance, Epidemiology and End Results (SEER) database in 40 years. The average age of onset is the 6th decade and there is a slight male predominance.2 Laryngeal lymphoma is even rarer in the pediatric age group. Ayyaswamy et al. found only 7 pediatric cases in the literature published between 1987-2022.3 We found 11 cases of laryngeal lymphoma diagnosed under 18 years of age in the English literature (Table I).3,4,7-14 Including our patients, there were a total of 9 male and 4 female cases (M/F: 2.25). The median age of patients was 10.5 (4-15) years. Hoarseness, dyspnea, and dysphagia are often the initial symptoms of laryngeal lymphoma.4 Neoplastic infiltration is mostly located in the supraglottic region and may extend to the glottis and subglottic area.3,4,7-14 Similar to the literature, the location in our cases was a supraglottic area. Immunodeficiency is one of the strongest risk factors for non-Hodgkin lymphoma especially originating from atypical sites.1,15 Diagnosis of immunodeficiency diseases was found in 2.5% of cases with non-Hodgkin lymphoma.16 Mayor et al. examined a group of 3658 patients with primary immunodeficiency and found that 171 (4.6%) cases had malignancy. The highest increase in cancer incidence was observed in lymphoma, with a 10-fold increase in men and an 8.34-fold increase in women.17 An article reporting an increased risk of EBV-associated lymphoproliferative diseases in STK4 deficiency, including our cases, has been published previously.18
| CT: chemotherapy, DLBCL: diffuse large B-cell lymphoma, EBER: Epstein-Barr encoding region, EBV: Epstein-Barr virus, F: female, IRF4: interferon regulatory factor 4, LBCL: large-B-cell lymphoma, M: male, mo: month, NA: not available, NHL: non-Hodgkin lymphoma, NOS: not otherwise specified, RT: radiotherapy, T-LBL: lymphoblastic T-cell lymphoma, wod: without disease, y: years. | |||||||
| Table I. Laryngeal lymphoma in children and adolescents reported in the literature. | |||||||
| Case | Ref / year | Age(y) / Sex | Site | Stage | Histopathologic subtype | Therapy | Follow-up |
| 1 | Wang7, 1972 | 14/M | Supraglottis (aryepiglottic fold, epiglottis, arytenoid, and the interior of the larynx) | I | NHL, NOS | RT | Alive wod, 8.5 y |
| 2 | Cohen8, 1987 | 4/F | Supraglottis, glottis, subglottis (entire supraglottic area, the right true vocal cord and false vocal cord, extending into the immediate subglottic area anteriorly along the anterior commissure) | I | NHL, NOS | CT, RT | Alive wod, 2 y |
| 3 | 9/F | Supraglottis and subglottis (epiglottis, glossoepiglottic ligaments, pharyngoepiglottic ligaments, arytenoids, aryepiglottic folds, false vocal cords, subglottic area) | I | NHL, NOS | CT, RT | Alive wod, 2 y | |
| 4 | Palenzula9, 2002 | 15/M | Supraglottis, glottis, subglottis (left supraglottis, left vocal cord, the subglottic area from the level of the cricoid cartilage to the left vocal cord) | I | DLBCL, EBER+ | CT, RT, tumor debulking by laser | Died |
| 5 | Naik4, 2012 | 10/M | Supraglottis, glottis (lesion in the right pyriform fossa extending to the right true and false vocal cords, aryepiglottic folds) | II | DLBCL | CT | Alive wod, 13 mo |
| 6 | Rodriguez10, 2014 | 8/M | Supraglottis, glottis (epiglottis, ventricular bands, glottis) | I | T-LBL | CT | Died, 16 mo |
| 7 | Martin11, 2017 | 14/F | Supraglottis (epiglottis, left arytenoid, post cricoid area) | I | LBCL, IRF4 (+) | Surgery, CT | Alive wod, 1 mo |
| 8 | Perez12, 2019 | 13/M | Supraglottis (right aryepiglottic fold, right supraglottic mass) | I | DLBCL | Surgery, CT | NA |
| 9 | Tsur13, 2021 | 13/M | Supraglottis, glottis (right false vocal cords, aryepiglottic folds, a cystic lesion on the right vocal cord bulging into the trachea and causing partial narrowing of the larynx) | II | DLBCL | CT | NA |
| 10 | Munjal14, 2021 | 7/M | Supraglottis, glottis (swelling of the glottis and supraglottic soft tissues with patent airway) | IV | DLBCL, EBV+ | Oncologic treatment | Died, within a few mo |
| 11 | Ayyaswamy3, 2022 | 9/M | Supraglottis (a single globular mass with an irregular surface arising from epiglottis extending till vallecula and base of tongue) | I | DLBCL | Surgery, CT | Alive wod, 12 mo |
| 12 | Present cases | 6/M | Supraglottis (left aryepiglottic fold) | II | Mature B-cell NHL | CT | Alive wod, 24 mo |
| 13 | Supraglottis (false vocal cords, arytenoid, aryepiglottic fold) | III | Mature B-cell NHL, EBER+ | CT | Alive wod, 30 mo | ||
A detailed family history and systemic examination are essential for the diagnosis of immunodeficiencies. These assessments help clinicians make a differential diagnosis, guide further testing, and improve prognosis by enabling timely treatment.19-21 Once a diagnosis has been made, genetic counseling and prenatal diagnosis may be offered to at-risk family members.22 Likewise, in our patient with progressive hoarseness, who was diagnosed with STK4 deficiency, it was determined that a 3x3 cm lymphadenopathy had developed in the right axilla during this period. Following diagnostic evaluation, the patient was diagnosed with stage IIIB diffuse large B-cell lymphoma. After chemotherapy, physical examination revealed regression of cervical and axillary lymph nodes, while a new lymphadenopathy measuring 1.5x1 cm was detected in the supraclavicular region. The biopsy performed due to suspicion of the disease was found to be consistent with lymphoma. The patient was started on chemotherapy once again and, during follow-up, underwent a bone marrow transplant and recovered. If a systemic evaluation had not been performed at the end of treatment, the disease could have progressed, and the patient could have died. In conclusion, we can conclude that a systemic examination is necessary for early diagnosis and treatment.
Although laryngeal lymphoma is rare, certain imaging features suggest this diagnosis. A homogeneously growing supraglottic tumor without central necrosis is the characteristic finding of lymphoma and it shows limited diffusion on diffusion-weighted imaging.23 There was no necrosis in the imaging findings of our first case, and a soft tissue lesion at the supraglottic level with diffusion restriction was detected in the diffusion-weighted images. Although imaging methods are helpful, a definitive diagnosis must be confirmed by biopsy.10
The current standard treatment of laryngeal lymphoma is chemotherapy, although some previous studies have shown that it can be successfully treated and remission can be achieved with radiotherapy.1 There is an increase in treatment success in high-risk patients with the addition of monoclonal antibodies such as rituximab to existing treatment regimens.24 Surgery may be necessary only in the case of laryngeal obstruction and massive bleeding.10 Among pediatric patients that we reviewed in the literature, four received radiotherapy before 2003, 11 received chemotherapy, and three underwent surgical procedures. Detail of the oncologic treatment was not present for one patient.
The prognosis of patients with laryngeal non-Hodgkin lymphoma is generally good. In the study of Zhao et al., the overall survival rate was 69.4%.22 One of the prognostic risk factors is the stage.24,25 All but one of the pediatric cases with laryngeal lymphoma in the literature had stage 1-2 disease. Another prognostic risk factor is the cellular subtype (B or T-cell). T cell subtype is associated with a worse prognosis.24,25 One patient among children diagnosed with laryngeal lymphoma in the literature was of the T cell subtype and died despite receiving chemotherapy.
The feature of this article as a case report naturally carries some limitations. However, we compiled and evaluated cases from the literature to reduce these limitations by increasing the number of cases.
In conclusion, symptoms such as dysphonia, dysphagia, and dyspnea in children are primarily attributed to infections, inflammatory conditions, or pubertal voice changes. Flexible or direct laryngoscopy should be performed in all patients with persistent dysphonia to exclude laryngeal tumors. Differential diagnosis in pediatric cases should include lymphoma.
Ethical approval
Written informed consent was obtained from the parents for this publication.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Roh JL, Huh J, Moon HN. Lymphomas of the head and neck in the pediatric population. Int J Pediatr Otorhinolaryngol 2007; 71: 1471-1477. https://doi.org/10.1016/j.ijporl.2007.06.004
- Hong SA, Tajudeen BA, Choi S, Husain IA. Epidemiology and prognostic indicators in laryngeal lymphoma: a population-based analysis. Laryngoscope 2018; 128: 2044-2049. https://doi.org/10.1002/lary.27074
- Ayyaswamy A, Saravanam PK, Sneha L, Sundaram S. Laryngeal lymphoma in a child - case report and review of literature. Iran J Otorhinolaryngol 2022; 34: 337-341. https://doi.org/10.22038/IJORL.2022.57663.2984
- Naik SM, Nanjundappa A, Halkud R, et al. Anaplastic lymphoma kinase-positive primary diffuse large B-cell lymphoma of the larynx: a rare clinical entity. Int J Phonosurg Laryngol 2012; 2: 57-61. https://doi.org/10.5005/jp-journals-10023-1038
- Aaron Silverman D, Wanner R, Walz P, O. Old M, R. Jatana K. Pediatric head and neck malignancies. In: Surgical management of head and neck pathologies. IntechOpen; 2021. Available at: http://doi.org/10.5772/intechopen.98316
- Arboleda LP, Hoffmann IL, Cardinalli IA, Santos-Silva AR, de Mendonça RM. Demographic and clinicopathologic distribution of head and neck malignant tumors in pediatric patients from a Brazilian population: a retrospective study. J Oral Pathol Med 2018; 47: 696-705. https://doi.org/10.1111/jop.12724
- Wang CC. Malignant lymphoma of the larynx. Laryngoscope 1972; 82: 97-100. https://doi.org/10.1002/lary.5540820113
- Cohen SR, Thompson JW, Siegel SE. Non-Hodgkin’s lymphoma of the larynx in children. Ann Otol Rhinol Laryngol 1987; 96: 357-361. https://doi.org/10.1177/000348948709600401
- Palenzuela G, Bernard F, Gardiner Q, Mondain M. Malignant B cell non-Hodgkin’s lymphoma of the larynx in children with Wiskott Aldrich syndrome. Int J Pediatr Otorhinolaryngol 2003; 67: 989-993. https://doi.org/10.1016/s0165-5876(03)00155-1
- Rodríguez H, Cuestas G, Bosaleh A, Passali D, Zubizarreta P. Primary laryngeal lymphoma in a child. Turk J Pediatr 2015; 57: 78-81.
- Martin A, Tu N, Duncan K, et al. Primary pediatric lymphoma of the larynx: an unusual presentation of a rare histologic subtype. Int J Pediatr Otorhinolaryngol Extra 2018; 19: 3-5. https://doi.org/10.1016/j.pedex.2017.10.001
- Perez PI, McIlwain W, Winters J, Dhesi M, Rogers DJ. A supraglottic mass in a pediatric patient: an unusual presentation of lymphoma. Ann Otol Rhinol Laryngol 2019; 128: 774-777. https://doi.org/10.1177/0003489419838544
- Tsur N, Apterman A, Sachs N, Abuhasira S, Hod R. A case of a 13-year-old adolescent with burkitt’s lymphoma presented with Dysphonia: common complaint yet uncommon presentation. Clin Lymphoma Myeloma Leuk 2021; 21: e598-e600. https://doi.org/10.1016/j.clml.2021.02.017
- Munjal T, Vukkadala N, Hazard FK, Meister KD. Next-generation sequencing as an auxiliary tool in pediatric laryngeal lymphoma diagnosis. Pediatrics 2021; 148: e2020047662. https://doi.org/10.1542/peds.2020-047662
- Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2007; 16: 405-408. https://doi.org/10.1158/1055-9965.EPI-06-1070
- Aricò M, Mussolin L, Carraro E, et al. Non-Hodgkin lymphoma in children with an associated inherited condition: a retrospective analysis of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP). Pediatr Blood Cancer 2015; 62: 1782-1789. https://doi.org/10.1002/pbc.25565
- Mayor PC, Eng KH, Singel KL, et al. Cancer in primary immunodeficiency diseases: cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol 2018; 141: 1028-1035. https://doi.org/10.1016/j.jaci.2017.05.024
- Saglam A, Cagdas D, Aydin B, et al. STK4 deficiency and EBV-associated lymphoproliferative disorders, emphasis on histomorphology, and review of literature. Virchows Arch 2022; 480: 393-401. https://doi.org/10.1007/s00428-021-03147-w
- Paul ME. Diagnosis of immunodeficiency: clinical clues and diagnostic tests. Curr Allergy Asthma Rep 2002; 2: 349-355. https://doi.org/10.1007/s11882-002-0066-2
- Klangkalya N, Fleisher TA, Rosenzweig SD. Diagnostic tests for primary immunodeficiency disorders: classic and genetic testing. Allergy Asthma Proc 2024; 45: 355-363. https://doi.org/10.2500/aap.2024.45.240051
- Bonilla FA, Geha RS. 12. Primary immunodeficiency diseases. J Allergy Clin Immunol 2003; 111: 571-581. https://doi.org/10.1067/mai.2003.86
- Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol 2010; 125: 297-305. https://doi.org/10.1016/j.jaci.2009.08.043
- Siddiqui NA, Branstetter BF, Hamilton BE, et al. Imaging characteristics of primary laryngeal lymphoma. AJNR Am J Neuroradiol 2010; 31: 1261-1265. https://doi.org/10.3174/ajnr.A2085
- Lombo C, Matos C, Fonseca R. Primary laryngeal lymphoma: a diagnostic challenge. Int J Otorhinolaryngol Head Neck Surg 2022; 8: 77-80 https://doi.org/10.18203/issn.2454-5929.ijohns20214853
- Zhao P, Zhou Y, Li J. Primary laryngeal lymphoma in China: a retrospective study of the last 25 years. J Laryngol Otol 2019; 133: 792-795. https://doi.org/10.1017/S0022215119001622
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















