Graphical Abstract
Abstract
Background. Enterobius vermicularis is a nematode that predominantly affects the pediatric population, particularly in families with school-aged children. While it typically causes intestinal symptoms, rare cases of extraintestinal involvement have been reported, including female genital tract involvement and complications such as appendicitis or enterocolitis. Perianal parasitic abscesses are also rare, with only a few cases reported in the literature.
Case Presentation. A 17-year-old female presented with abdominal pain during menstruation. Pelvic ultrasound revealed a septated cystic lesion located posterior to the uterus. Magnetic resonance imaging (MRI) demonstrated a perianal lesion with thick material, appearing iso- to hyperintense on both T1- and T2-weighted images. The lesion extended into the left gluteal muscles and showed peripheral contrast enhancement and diffusion restriction. Notably, there were no surrounding inflammatory changes, making an abscess diagnosis less likely. Surgical drainage revealed pus, and cytological analysis identified abundant parasitic oocytes consistent with E. vermicularis. The patient was treated with surgical drainage followed by pyrantel pamoate, resulting in near-complete resolution of the lesion at the one-month follow-up MRI. A literature review was also conducted to identify previously reported cases of parasitic abscesses and to explore the differential diagnoses of perianal cystic lesions.
Conclusions. Perianal abscesses due to parasitic infections are rare, particularly those caused solely by E. vermicularis. Given the high prevalence in childhood infestations, parasitic abscesses should be considered in the differential diagnosis of perianal collections in pediatric patients, especially in the absence of peripheral inflammatory signs.
Keywords: Enterobius vermicularis, parasitic abscess, perianal abscess, enterobiasis, oxyuriasis, pinworm
Introduction
The perianal, retrorectal, and presacral spaces are potential sites for various lesions in pediatric patients.1 While bacterial perianal abscesses are relatively common and often accompanied by signs of inflammation2, parasitic abscesses are exceedingly rare.
Enterobius vermicularis is a highly host-specific nematode, primarily infecting humans, with rare exceptions. The parasite predominates in the pediatric population, particularly among members of families with school-age children.3 While typically not associated with severe clinical manifestations, it can occasionally lead to complications such as abscess formation, ileocolitis, enterocutaneous fistula, pelvic inflammatory disease, appendicitis, tubo-ovarian abscess, and urinary tract infection.4
This report describes the clinical and radiological findings of a rare case of a perianal parasitic abscess associated with E. vermicularis in a 17-year-old girl with no underlying risk factors.
Case Presentation
A 17-year-old girl presented to the emergency department with abdominal pain during menstruation. A cystic lesion was identified on pelvic ultrasound, prompting a referral to our hospital for further evaluation and treatment. Physical and digital rectal examinations were unremarkable, with no detectable mass. She had no fever, no significant past medical history, and her blood tests were within normal limits.
A suprapubic pelvic ultrasound revealed a septated cystic lesion measuring 7.5 x 6.5 cm, located posterior to the uterus and left ovary. The uterus and ovaries appeared normal in size and morphology. Magnetic resonance imaging (MRI) demonstrated a cystic lesion extending from the left perianal area to the left gluteal region. The lesion appeared iso- to hyperintense on T1-weighted images and hyperintense on T2-weighted images, with thick material and a lobulated contour. The gluteal extension measured approximately 8 cm in length. No edematous signal changes, effusion, or perianal fistula suggestive of inflammation were observed (Fig. 1). The lesion showed diffusion restriction and exhibited peripheral contrast enhancement (Fig. 2).
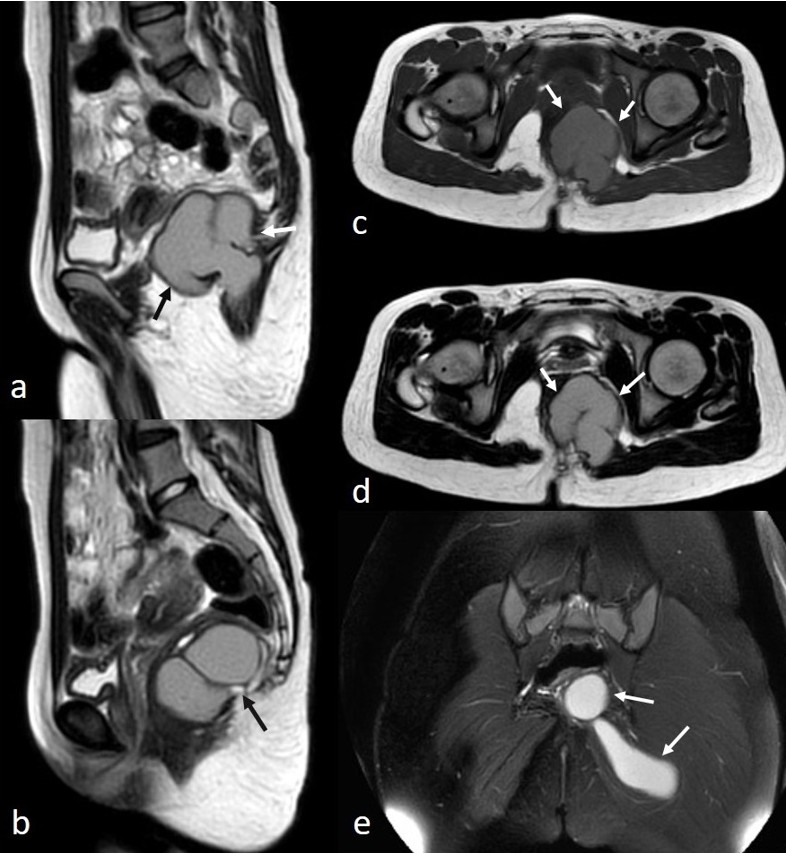
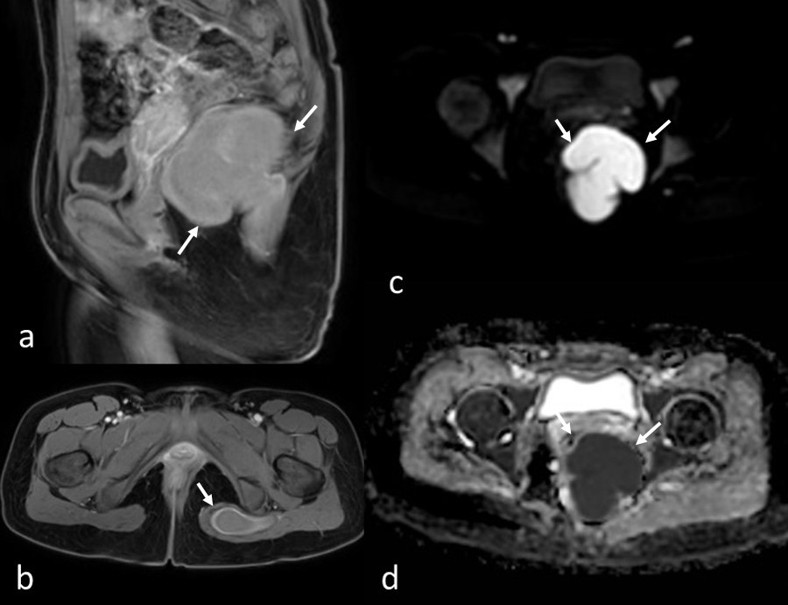
A bacterial abscess was deemed unlikely due to the absence of inflammatory features and rectal or gluteal pain. Imaging differential diagnoses included perianal developmental cysts, such as cystic teratomas and tailgut cysts, considering the patient’s age. During surgery, the patient was positioned prone, and a midline posterior sagittal incision was made. Exploration revealed an abscess extending from the presacral region to the left gluteal area, approximately 8-10 cm in size, without a distinct cystic structure. Based on intraoperative findings, the lesion was considered a perianal abscess. It was drained via simple puncture, and a drain was placed to allow continuous drainage.
The abscess culture showed no bacterial growth, but cytological analysis of the drainage fluid revealed abundant parasitic oocytes consistent with E. vermicularis, along with inflammatory cells. The lesion was diagnosed as a parasitic abscess, and the patient received pyrantel pamoate at 11 mg/kg/day for three days. Esophagogastroscopy and colonoscopy revealed mild hyperemia and increased vascularisation of the rectosigmoid colon, with no evidence of inflammatory bowel disease. Pathologic evaluation of the endoscopic biopsies showed lymphoid hyperplasia and edema-congestion, but no eosinophilia was reported. On the one-month postoperative follow-up MRI, the gluteal component was completely drained, with a small residual perirectal component observed, which continues to be monitored following medical treatment. Written informed consent was obtained from the patient and her parents for this report and accompanying images.
Discussion
We presented a rare case of a perianal parasitic abscess extending into gluteal muscles, notable for the absence of edematous signal changes, effusion, or fistula. This perianal parasitic abscess necessitated consideration of congenital lesions in the differential diagnosis, particularly cystic teratomas and tailgut cysts. Unlike our case, most reports and reviews in the literature highlight perianal cysts misdiagnosed as abscesses, perianal fistulae, pilonidal sinuses, and ovarian tumors.2,5,6 In our adolescent patient, cystic teratomas were prioritized in the differential as the most common presacral lesions in pediatric patients, alongside tailgut cysts, which predominantly affect young women. While these entities share overlapping clinical and radiological features, teratomas typically present earlier, during infancy or early childhood, compared to tailgut cysts.6
Perianal cysts can become infected, often making it challenging to distinguish between a perianal abscess and an infected perianal cyst.5,6 For example, Johnson et al. reported an adolescent female with a tailgut cyst misdiagnosed as a recurrent perianal fistula and pelvic abscess, underscoring the diagnostic difficulty. Features that should prompt consideration of a perianal cyst include a history of recurrent abscesses or multiple surgeries, the presence of a perianal or rectal sinus, failure to identify the infection’s usual source, and the presence of a postanal dimple, which is characteristic of tailgut cysts.6
Uncomplicated perianal cysts typically appear on imaging as well-defined, thin-walled, uni- or multilocular lesions. In contrast, infected cysts or abscesses in the perianal space present as thick-walled lesions with irregular margins and surrounding inflammatory changes.2,5
Transabdominal ultrasonography is often limited in differentiating perirectal cystic masses and abscesses from ovarian cysts, especially when the lesion is large or in an atypical location. In our case, a septated cystic lesion located posterior to the uterus and left ovary was detected during a suprapubic pelvic ultrasound. However, these findings were not definitive, prompting the recommendation for further MRI evaluation.
Computed tomography (CT) and MRI are valuable in assessing the nature of perianal lesions, providing information on wall thickness, lesion contents, and their relationship to adjacent structures, such as the ureter, urinary bladder, rectum, uterus, blood vessels, and sacral bone. CT findings of uncomplicated perianal cystic lesions typically show no contrast enhancement and hypoattenuation.2 In contrast, infected cysts or abscesses often present with thick, enhancing walls.2,5
MRI findings vary among perianal cysts and abscesses. Perirectal cysts are typically hypointense on T1-weighted images when purely cystic, but may show intermediate or high intensity depending on their content. Hyperintense T1 signals often result from mucoid or protein content in tailgut cysts or fatty content of teratomas/dermoid cysts, which disappear on T1-weighted fat-saturated images. The cysts are homogeneous and hyperintense on T2-weighted images.2 Abscesses exhibit diffusion restriction on MRI, with bacterial abscesses accompanied by peri-focal inflammatory changes. Similar findings may appear in infected perianal cysts, and diffusion restriction can also occur in uncomplicated perianal cysts like epidermoid cysts.5
In our case, the lack of surrounding inflammatory changes made a bacterial abscess or infected perirectal mass less likely. Well-defined margins, as well as high intensity on T1-weighted images, suggested perianal cysts, such as teratomas and tailgut cysts, as potential differential diagnoses. However, diffusion restriction and thick enhancing walls, consistent with perianal abscess formation, led to diagnostic uncertainty. There was no evidence of perianal fistula. The final cytological diagnosis of parasitic abscess confirmed that the iso-hyperintense appearance on T1-weighted images was due to the thick pus content of the abscess.
Perianal bacterial abscess formation and sepsis are relatively common in pediatric patients, especially in cases of Crohn’s disease, diabetes mellitus, immunodeficiency syndromes, trauma, and foreign bodies.7 Theories on the pathophysiology of primary perianal abscess in children suggest infection of anal glands, related to the entrapment of migratory cells from the urogenital sinus or abnormally wide crypts of Morgagni.
In a perianal parasitic abscess, E. vermicularis may contribute through several mechanisms. Mahomed et al. emphasized that although the precise mechanism of involvement remains unclear, one possibility is that the direct migration of the threadworms through intact mucosa into the anal glands, where they cause irritation and tissue inflammation, leading to occlusion and abscess formation. Another proposed pathway is invasion through pre-existing fistulae or glandular crypts, leading to inflammation with ova in the abscess wall or parasites present in the lumen, thereby contributing to abscess formation. Abscesses may therefore arise either from occlusion of the gland or fistulae openings, or from a localized reaction to deposited ova or parasites.7
Perianal abscesses caused by E. vermicularis can be symptomatic, as described by Durgun et al., who reported a 16-year-old female patient with no medical history, presenting with a perianal abscess, swelling, chills, and anal pain.8 Similarly, Shelat et al. described a 29-year-old male patient with a perianal abscess of E. vermicularis with swelling in the perianal region.9 However, perianal nodules or granulomas associated with E. vermicularis are generally asymptomatic and are typically surrounded by intact perianal skin. Gupta et al. noted that eosinophilia or positive stool tests are uncommon in such cases, with ova usually found in the content of the lesion since adult worms degenerate and become undetectable.10 In our case, the definitive diagnosis was made through cytological examination, which identified E. vermicularis oocytes.
Unlike the frequent occurrence of bacterial perianal abscesses, parasitic perirectal abscesses are exceedingly rare, with only a few cases reported in the literature. Sandhu et al. reported a recurrent perirectal abscess associated with Schistosoma in an HIV-infected adult male from an endemic area, presenting with sharp pain worsened during defecation, along with perirectal and lower sigmoid lymphadenopathies.11 Van Horn et al. reported a recurrent perianal abscess associated with copepods (Diacyclops thomasi) in an adult male with Crohn disease, who presented with severe perianal pain and purulent discharge. In that case, bacterial cultures were also positive, suggesting the parasitic involvement was secondary to bacterial abscess formation.12 In contrast, our case involves a parasitic perianal abscess extending into the gluteal muscles, without any risk factors, bacterial involvement, underlying causes, or perirectal symptoms. Echinoccoccus granulosus can rarely present primarily in the perianal or pelvic region. Abdalla et al. reported a 46-year-old female with a painless perianal mass, diagnosed as an ischioanal fossa hydatid cyst.13 Similarly, Nasrallah et al. described a 46-year-old male with refractory urticaria and bilateral pararectal partially calcified hydatid cysts (Table I).14
| Table I. Summary of published case reports on perianal parasitic infections. | |||||
| Authors | Age | Sex | Site | Parasite | Comorbidity |
| Mahomed et al.7 |
12 5 |
M M |
Perianal abscess Perianal abscess |
Enterobius vermicularis Enterobius vermicularis |
None None |
| Durgun et al.8 | 16 | F | Perianal abscess | Enterobius vermicularis | None |
| Shelat et al.9 | 29 | M | Perianal abscess | Enterobius vermicularis | None |
| Gupta et al.10 | 10 | M | Perianal nodule | Enterobius vermicularis | None |
| Sandhu et al.11 | 47 | M | Recurrent perianal abscess | Schistosoma | HIV |
| Van Horn et al.12 | 22 | M | Perianal abscess and fistula | Diacyclops thomasi | Crohn |
| Abdalla et al.13 | 46 | F | Perianal cyst | Echinoccoccus granulosus | None |
| Nasrallah et al.14 | 46 | M | Perianal calcified cyst | Echinococccus granulosus | None |
Perianal abscesses caused by parasitic infections, or presenting solely as parasitic abscesses, are rare. To the best of our knowledge, this is the first case report of a parasitic abscess extending into gluteal muscles associated with E. vermicularis in the pediatric population. As one of the most common parasitic agents causing an infestation in childhood, E. vermicularis should be considered as a potential cause of perirectal collections that occur without surrounding inflammatory changes. Accurate diagnosis relies on a combination of imaging and cytological examination. Optimal management requires surgical drainage complemented by targeted antiparasitic therapy, which is essential to achieve complete resolution, prevent recurrence, and avoid unnecessary interventions.
Ethical approval
Written informed consent was obtained from the patient and her parents for the publication of this report and the accompanying images.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Glasgow SC, Dietz DW. Retrorectal tumors. Clin Colon Rectal Surg 2006; 19: 61-68. https://doi.org/10.1055/s-2006-942346
- Dahan H, Arrivé L, Wendum D, Docou le Pointe H, Djouhri H, Tubiana JM. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics 2001; 21: 575-584. https://doi.org/10.1148/radiographics.21.3.g01ma13575
- Kucik CJ, Martin GL, Sortor BV. Common intestinal parasites. Am Fam Physician 2004; 69: 1161-1168.
- Ariyarathenam AV, Nachimuthu S, Tang TY, Courtney ED, Harris SA, Harris AM. Enterobius vermicularis infestation of the appendix and management at the time of laparoscopic appendectomy: case series and literature review. Int J Surg 2010; 8: 466-469. https://doi.org/10.1016/j.ijsu.2010.06.007
- Karn S, Huda F, David LE, et al. Recurrent retrorectal tailgut cyst mimicking deep pelvic abscess: A diagnostic dilemma. Radiol Case Rep 2022; 17: 2559-2562. https://doi.org/10.1016/j.radcr.2022.04.041
- Johnson KN, Young-Fadok TM, Carpentieri D, Acosta JM, Notrica DM. Case report: misdiagnosis of tailgut cyst presenting as recurrent perianal fistula with pelvic abscess. J Pediatr Surg 2013; 48: e33-e36. https://doi.org/10.1016/j.jpedsurg.2012.12.022
- Mahomed AA, MacKenzie RN, Carson LS, Jibril JA. Enterobius vermicularis and perianal sepsis in children. Pediatr Surg Int 2003; 19: 740-741. https://doi.org/10.1007/s00383-003-0971-z
- Durgun C, Alkan S. Rare gastrointestinal presentation of enterobius vermicularis: Anal abscess. D J Med Sci 2021; 7: 155-157. https://doi.org/10.5606/fng.btd.2021.25062
- Shelat VG, Ng SR, Sim R. Perianal abscess secondary to enterobius vermicularis infestation. Ann Acad Med Singap 2011; 40: 149.
- Gupta B, Jain S. Perianal nodule due to Enterobius vermicularis: Cytomorphological spectrum on fine needle aspiration cytology with a review of literature. Trop Parasitol 2018; 8: 53-55. https://doi.org/10.4103/tp.TP_33_17
- Sandhu G, Georgescu A, Korniyenko A, Florita C, Iuga A. Recurrent perirectal abscess. Am J Med 2010; 123: e13-e14. https://doi.org/10.1016/j.amjmed.2010.01.034
- Van Horn KG, Tatz JS, Li KI, Newman L, Wormser GP. Copepods associated with a perirectal abscess and copepod pseudo-outbreaks in stools for ova and parasite examinations. Diagn Microbiol Infect Dis 1992; 15: 561-565. https://doi.org/10.1016/0732-8893(92)90110-f
- Abdalla KM, Al Sharie AH, ALzu’bi YO, Daoud MN, Aleshawi AJ, Gharaibeh KI. Ischio-anal fossa hydatid cyst. Int J Infect Dis 2020; 92: 181-183. https://doi.org/10.1016/j.ijid.2020.01.015
- Nasrallah OG, ElRifai A, Sidani S, Jamali F. A rare case of bilateral para-rectal hydatid disease: a case report. Int J Surg Case Rep 2023; 108: 108388. https://doi.org/10.1016/j.ijscr.2023.108388
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.














