Graphical Abstract
Abstract
Background. Brucellosis is a zoonotic infection transmitted to humans by ingestion of contaminated unpasteurized dairy products or via direct or indirect contact with infected animals. It is characterized by nonspecific symptoms like fever and joint pain, and laboratory findings including anemia, leukopenia, thrombocytopenia, or rarely pancytopenia. Here we report a case of brucellosis with thrombocytopenia that did not improve despite anti-brucella treatment and required intravenous immunoglobulin treatment.
Case Presentation. A six-year-old boy from a brucellosis-endemic area presented with fever and fatigue. Initial laboratory tests showed moderate thrombocytopenia and a Brucella agglutination titer of 1/320. Brucella spp. was isolated from blood culture. Rifampicin, trimethoprim-sulfamethoxazole (TMP-SMX), and gentamicin treatment were given to the patient, and clinical improvement followed, with normalization of blood count. However, on day 10, severe thrombocytopenia with epistaxis and ecchymosis developed, suggestive of immune thrombocytopenia (ITP). Intravenous immunoglobulin at a dose of 1000 mg/kg was given, resulting in a rise in platelet count. The patient was discharged with rifampicin and TMP-SMX. During follow-up, his platelet levels returned to normal without the need for additional immunoglobulin, suggesting resolution of Brucella-related immune thrombocytopenia.
Conclusion. Brucellosis should be kept in mind in the differential diagnosis of thrombocytopenia in endemic regions. If there is no response to antimicrobial treatment in brucellosis patients presenting with thrombocytopenia, immune thrombocytopenia should be considered.
Keywords: Brucella spp., brucellosis, immune thrombocytopenic purpura, thrombocytopenia
Introduction
Brucellosis is a zoonotic infection transmitted to humans by ingestion of contaminated unpasteurized dairy products or via direct or indirect contact with infected animals. The causative agents are Brucella spp., which are facultative intracellular bacteria that can multiply within phagocytic cells.1 Brucellosis presents with nonspecific symptoms such as fever, malaise, anorexia, and arthralgia, and a broad spectrum of laboratory manifestations such as anemia, leukopenia, thrombocytopenia, or less frequently pancytopenia.2 Brucella-related thrombocytopenia is a rare and mild hematological finding, does not lead to bleeding complications, and tends to resolve with appropriate antibiotic therapy. However, in less common scenarios, an immune-mediated mechanism may cause a marked decrease in platelet counts, either at the onset of infection or during treatment, sometimes accompanied by clinically significant hemorrhagic symptoms.3
In this report, we present a case of brucellosis complicated by thrombocytopenia, which persisted despite anti-Brucella therapy, but subsequently improved following intravenous immunoglobulin (IVIG) treatment.
Case Presentation
A six-year-old boy applied to a local hospital with complaints of fever, fatigue and joint pain lasting for 3 days. His complete blood count revealed moderate thrombocytopenia. This is an area where brucellosis is endemic and it was reported that this patient had ingesting unpasteurized dairy products. The patient’s Brucella tube agglutination titer was positive with a titer of 1/320. The patient received rifampicin and trimethoprim-sulfamethoxazole (TMP-SMX) combination as anti-Brucella treatment regimen in the local hospital. Nevertheless, the patient’s fever persisted for 3 days. In addition, platelet values in his complete blood count gradually decreased, and along with the development of leukopenia and anemia. Therefore, the patient was referred to our hospital, where a pediatric infectious diseases and hematology specialist was also present, to exclude hematological malignancies and infection-induced hemophagocytic lymphohistiocytosis (HLH).
The patient had fever, fatigue, and joint pain for 6 days when he was admitted to our hospital. On physical examination, he had a fever (38.7 °C) and tachycardia (116/min, in sinus rhythm), other vital signs were normal. The spleen was palpated 4 cm below the left costal margin, and the liver was palpated 1.5 cm below the right costal margin. There was no lymphadenopathy, petechiae, or purpura. The rest of the physical examination was essentially unremarkable.
Laboratory test results were as follows: hemoglobin: 10.8 g/dL (normal range, 12-16 g/dL), white blood cell count (WBC): 2.22×103/μL (normal range, 4-10×103/μL), absolute neutrophil count (ANC): 0.86×103/μL (normal range, 1.5-8×103/μL), absolute lymphocyte count (ALC): 1.23×103/μL (normal range, 1-4.8×103/μL), platelet count: 60×103/μL (normal range, 150-400×103/μL), erythrocyte sedimentation rate: 44 mm/h (normal range 0-20 mm/h), C-reactive protein: 130 mg/L (normal range, 0-5 mg/L), ferritin: 681 ng/mL (normal range, 20-300 ng/mL). The peripheral blood smear made by direct finger-prick sampling revealed platelet clusters, typically forming groups of 5–10, with otherwise normal platelet morphology. No atypical cells or blasts were identified. Biochemical parameters in serum (alanine and aspartate transaminases, sodium, potassium, creatinine, uric acid) were within normal limits, and there was no hypertriglyceridemia. Laboratory findings were not fully supportive of infection-induced HLH. The patient fulfilled 3 (splenomegaly, cytopenias, hyperferritinemia) out of the 8 diagnostic criteria defined in the HLH-2004 guidelines.4 Although leukemia was considered in the differential diagnosis due to the patient’s fever, hepatosplenomegaly and cytopenia, Brucella was suspected based on the positive Brucella test results and the patient’s clinical history and symptoms, thus bone marrow aspiration was deemed unnecessary.
The patient was treated with rifampicin (20 mg/kg/d, p.o.), TMP-SMX (8 mg TMP/kg/d, p.o.), and gentamicin (7.5 mg/kg/d, i.v.). The patient’s fever decreased on the second day after his admission to our hospital. There were no signs of petechiae, purpura, or ecchymosis bleeding. Brucella spp. were isolated in his hemoculture. In the complete blood count taken on the 7th day of treatment in our facility, it was seen that the platelet count increased to 138×103/μL, neutropenia and lymphopenia had also improved.
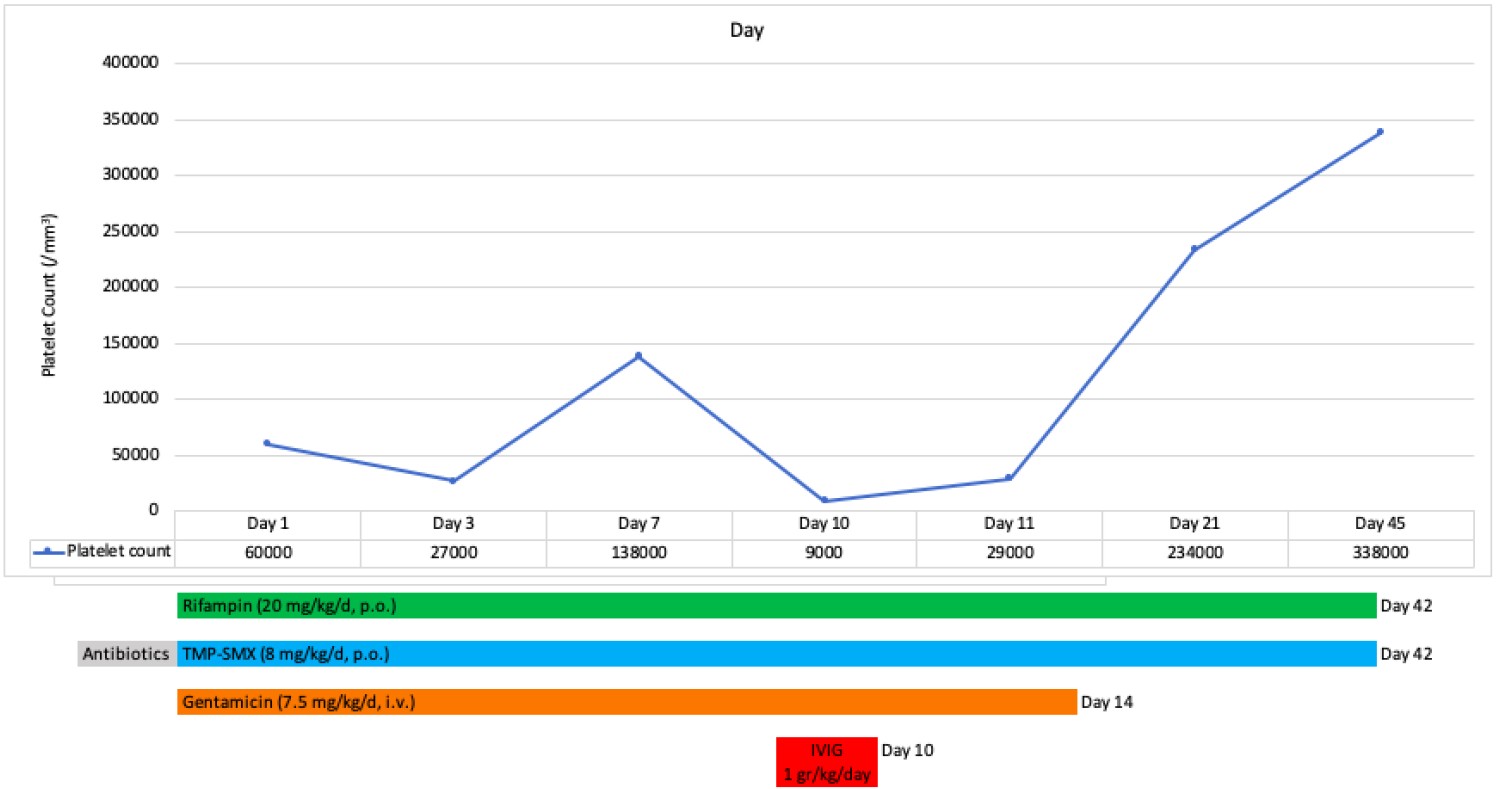
While the patient was under anti-Brucella treatment and his clinical findings were improving, his platelet count gradually decreased to 9×10³/μL on the 10th day of hospitalization in our facility. At that time, the complete blood count showed hemoglobin: 11.6 g/dL, WBC: 6.09×103/μL, ANC: 1.7 ×103/μL, and ALC: 3.78 ×103/μL. The peripheral blood smear obtained from a direct finger-prick showed no platelet clumping; instead, only occasional single platelets were observed. Again, no atypical cells or blasts were present. These findings effectively ruled out EDTA-dependent pseudothrombocytopenia. At the same time, the patient also had epistaxis and ecchymosis. He was afebrile, with a body temperature of 36.8 °C. Physical examination revealed multiple ecchymoses on the trunk and lower extremities. The spleen was palpable 3 cm below the left costal margin, and the liver was palpable 1 cm below the right costal margin. Other systemic examinations were within normal limits. Based on these new findings, the patient was considered to have immune thrombocytopenia. Along with the treatment against brucellosis, he was placed on intravenous immunoglobulin (IVIG) at 1 g/kg/day for one day. The day after IVIG platelet counts increased to 29 ×103/μL. Gentamicin was administered for 2 weeks. The patient was discharged on the 14th day with rifampicin and TMP-SMX as maintenance therapy, which were continued for a total of 6 weeks. Laboratory parameters during treatment and follow-up are presented in Table I.
| ALC, absolute lymphocyte count; ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; CRP, C-reactive protein; Hb, Hemoglobin; LDH, lactate dehydrogenase; PLT, platelets; WBC, white blood cell count. | |||||||
| Table I. Laboratory investigations and clinical findings during hospitalization and after discharge | |||||||
|
|
|
|
|
|
|
|
|
| Hb (g/dL) |
|
|
|
|
|
|
|
| WBC (×103/μL) |
|
|
|
|
|
|
|
| ANC (×103/μL) |
|
|
|
|
|
|
|
| ALC (×103/μL) |
|
|
|
|
|
|
|
| PLT (×103/μL) |
|
|
|
|
|
|
|
| AST (U/L) |
|
|
|
|
|
|
|
| ALT (U/L) |
|
|
|
|
|
|
|
| LDH (U/L) |
|
|
|
|
|
|
|
| Na (mEq/L) |
|
|
|
|
|
|
|
| Ferritin (ng/mL) |
|
|
|
|
|
||
| CRP (mg/L) |
|
|
|
|
|
|
|
| Body temperature (°C) |
|
|
|
|
|
|
|
| Epistaxis and ecchymosis |
|
|
|
|
|
|
|
In the outpatient clinic follow-ups, the patient’s platelet count increased to 338 ×103/μL. He did not require IVIG again during the follow-up. Chronological changes in platelet levels and the treatment timeline of the patient are shown in Fig. 1.
Informed consent was obtained from the patient’s parents for the publication.
Discussion
Brucellosis is a multisystemic disease that affects many systems, including the skeletal, central nervous, cardiovascular, and reticuloendothelial systems. Hematological abnormalities, such as anemia, lymphocytosis, thrombocytopenia and pancytopenia occurring in brucellosis in children have been reported in the literature.2,5 Anemia is more frequently associated with acute brucellosis, but pancytopenia and thrombocytopenia are less often seen. The incidence of thrombocytopenia in children with brucellosis varies between 5-14%.2,5 Many different mechanisms of thrombocytopenia observed during the clinical course of brucellosis have been described, including hypersplenism, bone marrow suppression, or immune-mediated.6-8
There is evidence that Brucella infection can prompt a systematic autoimmune response.9 This autoimmune stimulation may manifest as autoimmune hemolysis or platelet destruction. Several adult cases of immune thrombocytopenia associated with Brucella infection have been reported in the literature.9-11 Brucella-specific antibiotics and corticosteroids were started together for an 85-year-old woman patient with generalized purpura, rhinorrhagia, and severe thrombocytopenia.9 Another patient with immune thrombocytopenia due to brucellosis initially received Brucella-specific antibiotics only. Then corticosteroids were added to the treatment because thrombocytopenia did not improve.10 In another series of seven adult patients, thrombocytopenia resolved completely after appropriate antibiotic treatment for brucellosis was given to the patients. No additional treatment was required.11 It is noteworthy that in these reports involving adult patients, corticosteroid treatment was added to anti-Brucella antibiotics when thrombocytopenia did not improve or when purpura or bleeding was present.
In a study evaluating 14 children with brucellosis who presented with hematological manifestations, immune thrombocytopenia was detected in 5 of the patients (35.7%).6 The ages of these patients ranged from 3 to 10 years. The platelet counts of these patients ranged from 1 to 5 ×103/μL. In the case series, all patients with brucellosis-induced immune thrombocytopenia had severe thrombocytopenia with symptoms. Therefore, along with the anti-Brucella treatment, IVIG was given at 1 g/kg/day for 2 days.6 IVIG therapy exhibits a more rapid effect compared to steroids, as it inhibits the phagocytosis of antibody-coated platelets and reduces complement-mediated platelet destruction.12 In our patient, pancytopenia resolved after anti-Brucella antibiotic treatment was started. However, ecchymosis and epistaxis developed a few days later, and thrombocytopenia recurred. Due to the presence of mucosal bleeding, the need for a more rapid increase in platelet count was considered and IVIG therapy was administered. Similar to the cases in the literature, our patient benefited from IVIG treatment in terms of clinical and laboratory findings.
A review of pediatric cases of immune thrombocytopenia (ITP) associated with brucellosis in the literature reveals a notable diagnostic variability (Table II). In several cases, including our own, patients were initially diagnosed with brucellosis and received appropriate antimicrobial therapy; however, when thrombocytopenia persisted or worsened, a subsequent diagnosis of ITP was made.3,13 Conversely, there are also reports in which ITP was the initial presumed diagnosis, but brucellosis was later identified following further evaluation.14-16 In both groups of cases, favorable clinical outcomes were achieved with appropriate anti-Brucella therapy in combination with IVIG and/or corticosteroid treatment.
| F, female; ITP, immune thrombocytopenia; IVIG, intravenous immunoglobulin; M, male; N/A, Not applicable; TMP-SMX, Trimethoprim-Sulfamethoxazole; y, years. | ||||||||
| Table II. Reported cases of pediatric immune thrombocytopenia associated with brucellosis in literature. | ||||||||
| Article | Patient sex and age | Bleeding manifestations | Platelet count (103/μL) | Initial diagnosis | Time between diagnoses of brucellosis and ITP | Brucella treatment regimen (Duration) | ITP treatment regimen (Dose) | Response |
| Makis et al.3 |
F, 5.5 y |
Purpura, petechiae, gingival bleeding | 1 | Brusellosis | 3 day | TMP-SMX, rifampicin (6 weeks) |
IVIG (1 g/kg/day for 2 days) | Resolved |
| Şanal et al.13 |
M, 12 y |
Purpura, petechiae, mucosal bleeding, epistaxis | 4 | Brucellosis | 3 day | Doxycycline, rifampicin (6 weeks), gentamicin (N/A) |
IVIG (1 gr/kg/day) and methylprednisolone (30mg/kg) | Resolved |
| Sevinç et al.14 |
M, 16 y |
Purpura | 1 | ITP | 8 day | TMP-SMX, rifampicin, ciproflaxacin (N/A) | Methylprednisolone (30mg/kg) | Resolved |
| Tsirka et al.15 |
M, 11 y |
Petechiae, ecchymosis |
8 | ITP | 5 day | Doxycycline, rifampicin, gentamicin (N/A) | IVIG (N/A) | Resolved |
| Qiu et al.16 |
F, 2 y |
Purpura | 12 | ITP | 7 day | TMP-SMX, rifampicin (N/A) | IVIG (2 g/kg) | Resolved |
Conclusion
Brucellosis should be kept in mind in the differential diagnosis of thrombocytopenia in endemic regions. On the other hand, if there is no response to anti-Brucella treatment in brucellosis patients presenting with thrombocytopenia, immune mediated thrombocytopenia should be considered.
Ethical approval
Informed consent was obtained from the patient’s for the publication.
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis 2007; 7: 775-786. https://doi.org/10.1016/S1473-3099(07)70286-4
- al-Eissa Y, al-Nasser M. Haematological manifestations of childhood brucellosis. Infection 1993; 21: 23-26. https://doi.org/10.1007/BF01739305
- Makis A, Perogiannaki A, Chaliasos N. Severe thrombocytopenic purpura in a child with brucellosis: case presentation and review of the literature. Case Rep Infect Dis 2017; 2017: 3416857. https://doi.org/10.1155/2017/3416857
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48: 124-131. https://doi.org/10.1002/pbc.21039
- Justman N, Fruchtman Y, Greenberg D, Ben-Shimol S. Hematologic manifestations of brucellosis in children. Pediatr Infect Dis J 2018; 37: 586-591. https://doi.org/10.1097/INF.0000000000001900
- Citak EC, Citak FE, Tanyeri B, Arman D. Hematologic manifestations of brucellosis in children: 5 years experience of an Anatolian center. J Pediatr Hematol Oncol 2010; 32: 137-140. https://doi.org/10.1097/MPH.0b013e3181ced382
- Young EJ, Tarry A, Genta RM, Ayden N, Gotuzzo E. Thrombocytopenic purpura associated with brucellosis: report of 2 cases and literature review. Clin Infect Dis 2000; 31: 904-909. https://doi.org/10.1086/318129
- Yildirmak Y, Palanduz A, Telhan L, Arapoglu M, Kayaalp N. Bone marrow hypoplasia during Brucella infection. J Pediatr Hematol Oncol 2003; 25: 63-64. https://doi.org/10.1097/00043426-200301000-00012
- Pappas G, Kitsanou M, Christou L, Tsianos E. Immune thrombocytopenia attributed to brucellosis and other mechanisms of Brucella-induced thrombocytopenia. Am J Hematol 2004; 75: 139-141. https://doi.org/10.1002/ajh.10473
- Guzel Tunccan O, Dizbay M, Senol E, Aki Z, Ozdemir K. Isolated severe immune thrombocytopenia due to acute brucellosis. Indian J Hematol Blood Transfus 2014; 30: 27-29. https://doi.org/10.1007/s12288-012-0222-3
- Yilmaz M, Tiryaki O, Namiduru M, et al. Brucellosis-induced immune thrombocytopenia mimicking ITP: a report of seven cases. Int J Lab Hematol 2007; 29: 442-445. https://doi.org/10.1111/j.1365-2257.2006.00880.x
- Matzdorff A, Alesci SR, Gebhart J, et al. Expert report on immune thrombocytopenia: current diagnostics and treatment - recommendations from an expert group from Austria, Germany, and Switzerland. Oncol Res Treat 2023; 46(Suppl 2): 5-44. https://doi.org/10.1159/000529662
- Şanal KU, Metin Akcan Ö, Çopur A, Tokgöz H. Severe thrombocytopenia associated with brucellosis. J Pediatr Inf 2024; 18: e189-e191. https://doi.org/10.5578/ced.20240307
- Sevinc A, Kutlu NO, Kuku I, Ozgen U, Aydogdu I, Soylu H. Severe epistaxis in brucellosis-induced isolated thrombocytopenia: a report of two cases. Clin Lab Haematol 2000; 22: 373-375. https://doi.org/10.1046/j.1365-2257.2000.00334.x
- Tsirka A, Markesinis I, Getsi V, Chaloulou S. Severe thrombocytopenic purpura due to brucellosis. Scand J Infect Dis 2002; 34: 535-536. https://doi.org/10.1080/003655402320208785
- Qiu Z, Yang F, Zhang S. Immune thrombocytopenic purpura and its rare association with a brucella infection: a case report. Cureus 2022; 14: e30049. https://doi.org/10.7759/cureus.30049
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















