Graphical Abstract
Abstract
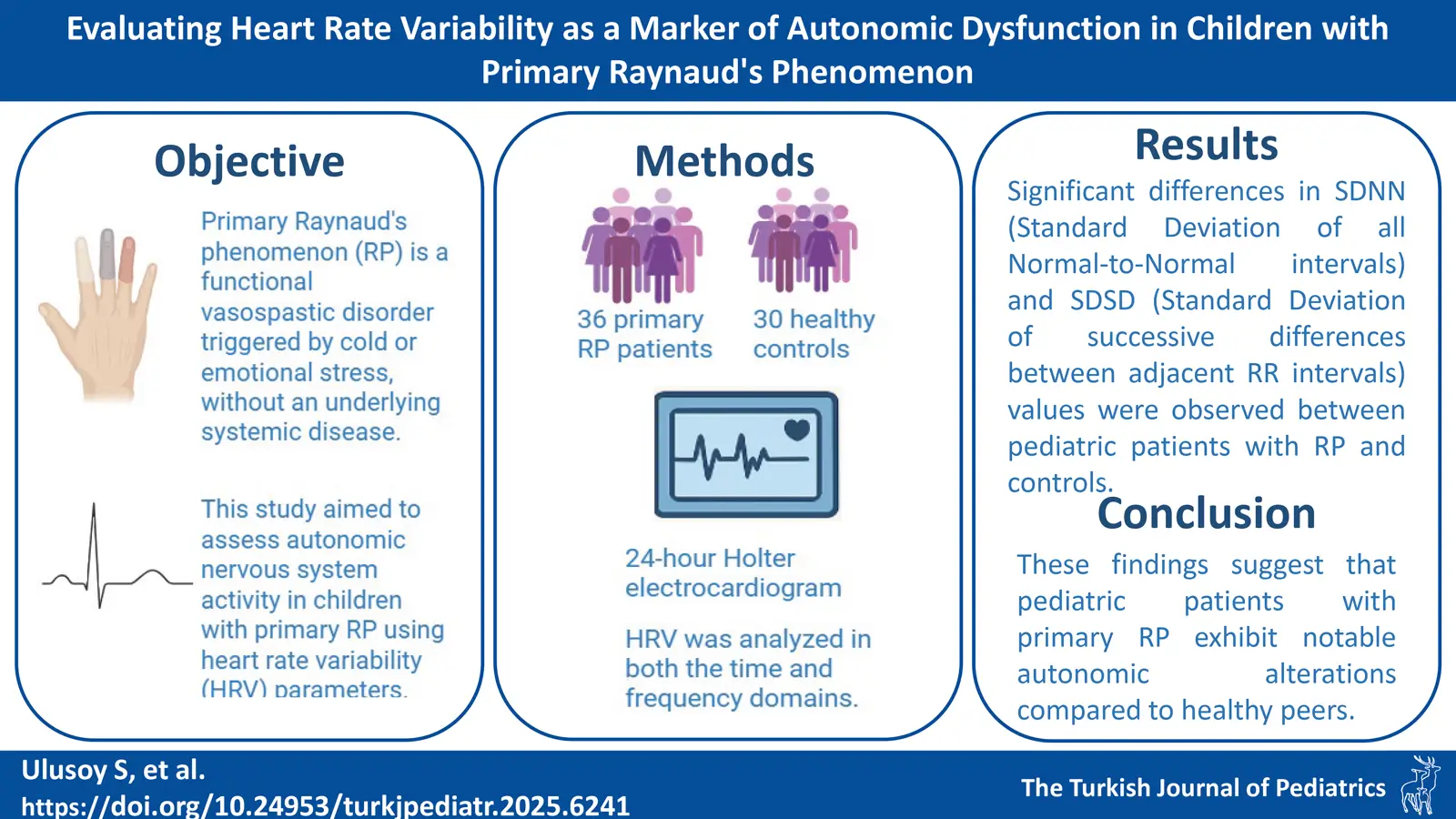
Background. Primary Raynaud’s phenomenon (RP) is a functional vasospastic disorder triggered by cold or emotional stress, often occurring without an underlying systemic disease. As autonomic dysfunction is thought to contribute to RP pathogenesis, heart rate variability (HRV) analysis may provide insights into underlying mechanisms. This study aimed to assess autonomic nervous system activity in children with primary RP using HRV parameters.
Methods. The study included 36 primary RP patients (0–18 years) and age- and gender-matched 30 healthy controls with normal 24-hour Holter electrocardiograms (ECG). Data on demographics, laboratory results, 24-hour Holter ECG, capillaroscopy, and treatment were collected. HRV was analyzed in both the time and frequency domains.
Results. In the patient group, 11 (30.4%) were male, and 25 (69.6%) were female, with a median age of 15 (8–18) years. Symptom onset occurred at a median age of 14. The attack patterns were biphasic in 36.1% of patients, triphasic in 30.6%, and monophasic in 33.3%. Capillaroscopy was normal in 16 (44.4%) patients, with minor changes in 20 (55.6%). Six (16.6%) patients had positive antinuclear antibody (ANA) with no autoimmune disease diagnoses. Holter ECG monitoring results were compared with those of healthy controls (median age 15 years), showing significant differences in standard deviation of all normal-to-normal intervals (SDNN) and standard deviation of successive differences between adjacent RR intervals (SDSD) between primary RP patients and controls, but no differences in root mean square of successive differences (RMSSD) or HRV index values.
Conclusion. Pediatric patients with primary RP showed significant autonomic changes compared to controls, though it remains unclear if these changes favor sympathetic or parasympathetic pathways. Further multicenter, prospective studies are needed to clarify these findings.
Keywords: Raynaud’s phenomenon, heart rate variability, autonomic nervous system activity, antinuclear antibody, capillaroscopy
Introduction
Raynaud’s phenomenon (RP) is characterized by transient vasospastic episodes in peripheral blood vessels, leading to a characteristic three-phase color change—pallor, cyanosis, and hyperemia—typically affecting the extremities.1 In some cases, the tip of the nose and the ears can also be involved.1 While data on the prevalence of RP in childhood is scarce, a study by Jones et al.2 found a prevalence of 18% in girls and 12% in boys aged 12–15 years. Because RP episodes are frequently triggered by cold and emotional stress—both of which involve autonomic nervous system activity—it has been suggested that dysregulation of autonomic function may play a role in the pathophysiology of RP.1,2
Heart rate variability (HRV) reflects fluctuations in the time intervals between consecutive heartbeats, and can be measured over short-term (e.g., 5 minutes) or long-term (e.g., 24 hours) electrocardiogram (ECG) recordings. HRV analysis provides a non-invasive window into autonomic nervous system function, and is typically expressed using time-domain or frequency-domain indices.3,4 For example, time-domain indices such as standard deviation of all normal-to-normal intervals (SDNN) or root mean square of successive differences (RMSSD) reflect beat-to-beat variability, while frequency-domain indices capture oscillatory patterns associated with sympathetic and parasympathetic activity.5 Specifically, the high-frequency (HF) component is linked to parasympathetic activity, while the low-frequency (LF) component reflects a mix of sympathetic and parasympathetic influences.4,6 Given this background on HRV as a marker of autonomic regulation, it is relevant to explore how these indices behave in patients with RP. Studies in adults with primary RP have demonstrated signs of autonomic imbalance, including increased sympathetic activity, reduced parasympathetic tone, and altered HRV parameters compared to healthy controls.7,8 These findings suggest that autonomic dysfunction may contribute to the pathogenesis of RP. However, studies on this topic in childhood are rare. A pediatric study evaluating primary RF by Oflaz et al.9 demonstrated abnormalities in time-domain HRV parameters, including SDNN, SDNN index (mean of the SDNN for all 5-min segments; SDNNi), standard deviation of the average NN intervals (SDANN), RMSSD, percentage of NN50 (pNN50), and the triangular index. Exploring HRV changes in children with primary RP could help clarify whether similar autonomic disturbances occur early in the disease course. Elucidating autonomic nervous system involvement in primary RP during childhood could improve our understanding of the disease’s pathophysiology and potentially guide management, since autonomic markers like HRV might eventually serve as early indicators of dysfunction. Given the role of the autonomic nervous system in mediating vascular responses to stress, we hypothesized that children with primary RP may exhibit measurable autonomic dysfunction. By analyzing HRV, which is a non-invasive marker of autonomic activity, this study seeks to fill a knowledge gap in pediatric RP.
This study aimed to assess heart rate variability in children with primary RP compared to age-matched healthy peers to determine whether RP in childhood is associated with autonomic nervous system dysfunction by evaluating the time—and frequency-dependent HRV in children diagnosed with primary RP.
Materials and Methods
Patients aged 0–18 years, diagnosed with RP and followed up at the Pediatric Rheumatology Outpatient Clinic, were enrolled in the study. Initially, patients with primary RP were documented. The diagnosis of primary RP was based on clinical assessment and fulfilled the International Consensus Criteria for Raynaud’s Phenomenon, which include episodic, symmetrical, and reversible color changes of the extremities in response to cold exposure or emotional stress, in the absence of an underlying systemic disease.10 In all cases, a comprehensive work-up including medical history, physical examination, immunologic screening, and echocardiography was performed to exclude secondary RP causes. Subsequently, the medical records of these patients were reviewed to collect data on demographic characteristics, the time of diagnosis, symptoms, comorbidities, treatments, clinical course, laboratory findings, and capillaroscopic evaluations. Capillaroscopic patterns were classified according to the criteria proposed by Ingegnoli et al.11, including normal, minor abnormalities, major abnormalities, and scleroderma pattern. Minor abnormalities were defined as a capillary density of 6–8/mm with <10% elongated loops, <50% tortuous loops, all arranged in parallel rows, and no hemorrhages. Evaluations were performed by an experienced pediatric rheumatologist using standard magnification videocapillaroscopy. Finally, heart rhythm analysis was performed on data from 24-hour three-channel Holter ECG monitoring before initiation of medical therapy for RP. All Holter recordings were obtained during asymptomatic periods, as confirmed by patient reports and absence of active color changes during monitoring. This approach was chosen to evaluate baseline autonomic function independent of acute vasoactive fluctuations during RP episodes.
Exclusion criteria included the presence of connective tissue diseases, conditions like skin ulceration, telangiectasia, muscle weakness, scleroderma, and factors affecting heart rate such as hypothermia, hyperthermia, chronic diseases (e.g., diabetes, hypertension), structural heart disease, or the use of medications affecting heart rhythm.
Patients were advised not to engage in strenuous exercise during the 24-hour Holter ECG monitoring. For the HRV analysis, all patients’ Holter recordings were manually assessed to exclude artifacts and ectopic beats. Only recordings with at least 85% of data composed of normal R-wave morphology were included. HRV parameters were automatically extracted using dedicated Holter data processing software. Time-domain measurements included SDNN, standard deviation of successive differences between adjacent RR intervals (SDSD), SDANN and RMSSD. Frequency-domain measurements were presented, including HF band, LF band, and LF/HF ratio.4 Time-domain parameters quantify the variability in successive RR intervals over time. SDNN reflects the overall variability and is influenced by both sympathetic and parasympathetic activity. SDSD and RMSSD primarily reflect parasympathetic (vagal) activity. RMSSD is calculated by determining successive RR interval differences, squaring each, averaging the squared values, and taking the square root of this average.4,5
Frequency-domain analysis involves spectral decomposition of RR interval time series into frequency bands. The high-frequency (HF: 0.15–0.40 Hz) band reflects parasympathetic activity, especially associated with respiratory sinus arrhythmia. The low-frequency (LF: 0.04–0.15 Hz) band reflects a combination of sympathetic and parasympathetic modulation. The LF/HF ratio is used as an indicator of sympathovagal balance. Very low frequency (VLF) and ultra-low frequency (ULF) bands are also detectable but were not analyzed in this study due to methodological limitations.4,6,12 The LF band (0.04–0.15 Hz) is comprised of rhythms with periods between 7 and 25 s. The HF or respiratory band (0.15–0.40 Hz) is comprised of rhythms with periods between 7 and 2.5 s. HRV parameters were extracted using the Spacelabs Healthcare Pathfinder SL software (Spacelabs Healthcare Inc., Snoqualmie, WA, USA).
The control group consisted of age- and gender-matched healthy children with normal 24-hour Holter ECG recordings.
The Kocaeli University Ethics Committee received approval under the approval number on December 13th, 2022. (GOKAEK-2022/20.23).
Statistical analysis
The study employed both parametric and non-parametric statistical analyses, utilizing IBM SPSS 20.0 for data processing. The normality of continuous variables was assessed using the Kolmogorov-Smirnov test. Variables following a normal distribution were expressed as mean ± standard deviation, while those not conforming to normal distribution were reported as median values with minimum and maximum. Categorical data were summarized as frequencies and percentages. Group comparisons of continuous variables were conducted using the Student T-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. A two-tailed p-value of <0.05 was considered statistically significant for all analyses, with a 5% threshold set for significance testing.
Results
Study group
A total of 36 patients were evaluated of whom 11 (30.4%) were male and 25 (69.6%) were female. The median age of symptom onset was 14 (5–16) years, the median age at diagnosis was 15 (7–17) years, and the median age at study enrollment was 15 (8–18) years. Three patients (8.3%) had a family history of RP, and the rate of consanguineous marriage among the parents was 13.9% (n=5).
The control group consisted of 30 healthy volunteers including, 20 females (66.7%) and 10 males (33.3%). No significant difference in gender distribution was observed when compared to the patient group (p=0.894). The median age of the control group was 15 (9–18) years with no significant age difference compared to the patient group (p=0.546). Body mass index (BMI) was not different between groups (The patient group: 19.9±4 kg/m2, The control group: 20.1±3.2, p=0.88).
Clinical and laboratory characteristics of patients
Regarding clinical findings, 13 (36.1%) patients exhibited biphasic symptoms, 11 (30.6%) patients presented with triphasic symptoms, and 12 (33.3%) patients had monophasic symptoms. Discoloration patterns were observed as follows: cyanosis in 33 (91.7%) patients, pallor in 22 (61.1%) patients, and redness in 21 (58.3%) patients. None of the patients developed digital ulcers, though 7 patients (19.4%) reported experiencing sweating. RP was noted in the upper extremities of 34 (94.4%) patients and in the lower extremities of 19 (52.8%) patients. Seventeen (47.2%) patients had symptoms affecting both upper and lower extremities, while 2 (5.6%) patients had signs restricted to the lower extremities and 17 (47.2%) patients had symptoms confined to the upper extremities. Additionally, RP symptoms were reported in the ears of 3 (8.3%) patients and in the nose of 2 (5.6%) patients.
Seasonal triggers revealed that symptoms first appeared in 16 (44.4%) patients during the winter, 15 (41.7%) in the autumn, 2 (5.6%) in the spring, and 3 (8.3%) in the summer. Symptom exacerbation was noted in 31 (86.1%) patients during the winter, while 5 (13.9%) patients experienced symptoms consistently throughout all seasons. Stress was identified as an aggravating factor in 16 (44.4%) patients.
Laboratory investigations revealed a median white blood cell count of 7245/mm³ (4150–11060), hemoglobin level of 13 g/dL (10.7–16.7), and platelet count of 265,500/mm³ (177,000–460,000). C-reactive protein (CRP) levels were within normal limits for all patients, although 4 patients had elevated erythrocyte sedimentation rate (ESR) levels. The median was 5.5 mm/h (2–52). Complement levels were within normal ranges for all patients, with a median complement (C) 3 level of 1.1 (0.9–2) and a median C4 level of 0.2 (0.1–0.5). Six (16.6%) patients tested positive for antinuclear antibodies (ANA), though all were negative for anti–double-stranded DNA antibody (anti-dsDNA). No significant antibody positivity was observed in the extractable nuclear antigen (ENA) panel or antiphospholipid antibody tests. None of the patients fulfilled the diagnostic criteria for autoimmune diseases.
Capillaroscopic examination revealed normal findings in 16 (44.4%) patients, whereas 20 (55.6%) exhibited minor abnormalities, such as tortuous capillaries.
In terms of treatment, all patients were initially advised on preventive measures. Medical treatment was administered to 15 patients, with 12 receiving acetylsalicylic acid, 5 of whom were also prescribed pentoxifylline concurrently, and 3 patients were started on nifedipine.
Evaluation of heart rate variability
Holter ECG analysis of the patients with primary RP revealed statistically significant differences in SDNN and SDSD when compared to healthy controls. However, no significant differences were found in RMSSD and HRV index values between the groups (Table I). Minimum-maximum heart rate and average heart rate were not statistically different between the groups. Although HF and LF band powers were lower and LF/HF was higher in patients with RP than in the control group, there was no statistically significant difference between the groups. In the patient group, when comparing 24-hour Holter monitoring parameters based on the presence of capillaroscopy findings, there were no significant differences between patients with capillaroscopic abnormalities and those with normal capillaroscopy findings.
| HF: high frequency, HRV: heart rate variability, LF: low frequency, SDNN: standard deviation of all normal-to-normal intervals, SDSD: standard deviation of successive differences between adjacent RR intervals, RMSSD: root mean square of successive differences. | |||
| Table I. Comparison of 24-hour Holter electrocardiogram monitoring analyses between the patient and control groups | |||
|
|
|
|
|
| Heart rate | |||
| Average of heart rate, bpm |
|
|
|
| Minimum heart rate, bpm |
|
|
|
| Maximum heart rate, bpm |
|
|
|
| Time-domain HRV parameters | |||
| SDNN, ms |
|
|
|
| SDSD, ms |
|
|
|
| RMSSD, ms |
|
|
|
| HRV index |
|
|
|
| Frequency-domain HRV parameters | |||
| HF band, ms2 |
|
|
|
| LF band, ms2 |
|
|
|
| LF/HF ratio |
|
|
|
Discussion
This study found that children with primary RP exhibited significantly lower SDNN and SDSD values than healthy controls, suggesting altered autonomic nervous system function. These findings highlight a potential role for HRV analysis in identifying early autonomic dysregulation in pediatric RP.
Raynaud’s phenomenon typically manifests with a triphasic pattern of pallor, cyanosis, and redness. However, some patients may present with monophasic or biphasic patterns. Maricq et al.13 reported that only 1% of patients exhibited a triphasic pattern. Furthermore, Nigrovic et al.14 found that only 24% of patients with primary RP and 19% with secondary RP displayed the classic triphasic pattern, with nearly half of the cases showing a monophasic pattern. In our study, 13 patients (36.1%) exhibited biphasic symptoms, 11 patients (30.6%) presented with triphasic symptoms, and 12 patients (33.3%) had monophasic symptoms.
Raynaud’s phenomenon predominantly affects the distal extremities. In a multicenter cohort study by Falcini et al.15, 99% of RP patients had upper extremities involvement with 37.2% also displaying symptoms in the lower extremities and face. In our study, RP was observed in the upper extremities of 34 patients (94.4%) and in the lower extremities of 19 patients (52.8%). Seventeen patients (47.2%) exhibited symptoms in both the upper and lower extremities. Additionally, RP symptoms were reported in the ears of 3 patients (8.3%) and in the nose of 2 patients (5.6%).
In terms of capillaroscopic findings, limited research exists on pediatric patients with primary RP. Pavlov-Dolijanovic et al.16 reported that 80% (173 of 191) of patients with primary RP had normal capillaroscopic findings, while 20% showed nonspecific changes. Importantly, no reduction in capillary density or enlargement in capillary size was observed in any of the primary RP cases. In our study, capillaroscopic examination showed normal findings in 16 patients (44.4%), while 20 patients (55.6%) displayed minor abnormalities, including tortuous capillaries. In the patient group, 24-hour Holter ECG monitoring parameters showed no significant differences between those with capillaroscopic abnormalities and those with normal findings. This is expected, as all patients had primary RP, a functional vasospastic condition without structural microvascular damage. Unlike secondary RP, minor capillaroscopic changes in primary RP are unlikely to affect autonomic function.
Previous studies considered normal ESR and ANA levels essential for a primary RF diagnosis. However, in the newly established consensus criteria, the requirement for negative ESR has been fully eliminated, and the negative ANA criterion has been relaxed to allow for either negative or low-titer ANA (e.g., 1:40 by indirect immunofluorescence).10 In the present study, six patients (16.6%) were positive for low-titer ANA, but none of them had other evidence of systemic connective tissue disease.
Heart rate variability parameters are significantly influenced by average heart rate. Therefore, in the interpretation of HRV in different groups, the average heart rate must be considered.17 Both groups in our study had similar average heart rate (p=0.914). Although there is still controversy over how sympathetic and vagal stimulus affect the LF band formation, the LF band likely represents the balance between the sympathetic and vagal stimuli. With predominant sympathetic excitation, a decrease in the LF component is observed. It has been shown that during exercise LF band is markedly reduced. The HF or respiratory band is influenced by breathing from 9 to 24 bpm.4 An overactive local vasoconstrictive response in the digital cutaneous vessels is the fundamental starting point of the primary RP. Defective sympathetic and parasympathetic responses localized in the digital cutaneous vessels might be implicated in the pathogenesis of primary RF. Some adult studies investigating HRV parameters in primary RP found a slight sympathetic predominance with decreased LF band power.7,18 Although it did not reach statistical significance, our study showed a slight decrease in LF band power in the patient group.
Both sympathetic and parasympathetic activity contribute to SDNN, and it is highly correlated with VLF and LF band power. The SDNN is more accurate when calculated over 24 h than during the shorter periods (5 min). The SDNN is a significant predictor of cardiac risk in adults with cardiac disease when recorded over a 24 h period. SDNN values predict both morbidity and mortality.3 Based on 24 h monitoring, patients with SDNN values below 50 ms are classified as unhealthy, 50-100 ms have compromised health, and above 100 ms are healthy.3 Both groups in our study had SDNN values above 100 ms, but patients with primary RP had lower SDNN value than the control group (p=0.04). Low SDNN values in patients with primary RP means that the ability of the sympathetic and parasympathetic stimuli to produce large fluctuations in heart rate (i.e. RR interval) is diminished in this group of patients. Low SDNN values have consistently been demonstrated in adult patients with primary and secondary RP.19 Furthermore, the SDSD is an index of successive beat-to-beat variability determined largely by autonomic influences on the heart. Therefore, decreased SDSD can be used as a sign of impaired and deranged sympathetic and parasympathetic activation. Oflaz et al.9 evaluated autonomic nervous system function in 32 children with primary RP using time-domain HRV analysis, including parameters such as SDNN, SDNNi, SDANN, RMSSD, pNN50, and the triangular index. These results were compared with those of 30 healthy controls. The study found that children with RP exhibited significantly higher average heart rates and significantly lower values in several HRV measures (SDNN, SDNNi, SDANN, and triangular index) compared to the control group. The authors concluded that these findings indicate a shift toward sympathetic dominance and reduced autonomic modulation in children with PRP. Both our study and the study by Oflaz et al.9 were conducted in pediatric populations; however, there are notable differences in the reported HRV parameters. While we observed significant alterations in SDNN and SDSD in primary RP patients compared to controls, we did not find significant differences in RMSSD or triangular index. Although the age and gender distribution and the measurement methodology were similar between these two studies, differences in disease duration or severity may account for the discrepancy in findings. While the study by Oflaz et al.9 does not provide detailed information on these parameters, our patient group had a relatively short disease duration and exhibited mild clinical symptoms. This may explain the more limited alterations observed in HRV parameters in our study.
The lack of significant changes in RMSSD or frequency-domain parameters may suggest that mild autonomic dysfunction in pediatric RP may selectively affect short-term time-domain parameters. Given the predominant parasympathetic mediation of SDSD and RMSSD, the selective decrease in SDSD might reflect subtle alterations in vagal tone, which is not sufficient to affect all indices. These findings may indicate early or partial autonomic dysregulation in pediatric RP, in contrast to more pronounced findings reported in adult populations.
A recent study exploring autonomic-targeted interventions, such as neurofascial vascular training, demonstrated symptomatic improvement in patients with primary RP through autonomic nervous system stimulation.20 A detailed evaluation of HRV in children with primary RP may assist in identifying early functional alterations in the autonomic nervous system. This could be essential for developing non-pharmacological approaches that target autonomic modulation in this population.
The main limitation of our study is its single-center design, which limits the generalizability of the findings to a specific population rather than the broader population. Due to the rarity of primary RP in childhood, no formal power analysis was performed; however, including all eligible patients provides valuable preliminary data in this underexplored area. The relatively small sample size may reduce the ability to detect more subtle differences in HRV parameters and should be considered when interpreting borderline or non-significant findings. Additionally, the cross-sectional design prevents longitudinal follow-up and further evaluation of patient outcomes over time. However, our study has some strengths that should be emphasized. Notably, it included frequency-domain HRV analysis and nailfold capillaroscopy, which allowed for a more comprehensive evaluation of both autonomic function and microvascular involvement. Although the differences between primary RP patients and healthy controls were not statistically significant in these parameters, the use of these advanced methodologies enhances the depth and quality of the assessment.
In conclusion, pediatric patients with primary RP exhibited significant alterations in autonomic nervous system activity compared to healthy controls, particularly reflected by reduced SDNN and SDSD values. These changes suggest subclinical autonomic dysregulation, which may have implications for early identification or monitoring of affected children. Although the specific balance between sympathetic and parasympathetic influence remains unclear, identifying the predominant autonomic pathway could guide future diagnostic or therapeutic strategies. The lack of significant changes in RMSSD or frequency-domain parameters may indicate that dysregulation is mild or selectively affects short-term time-domain indices. Given the rarity of pediatric RP, our findings provide valuable preliminary insight into autonomic function in this population. Future multicenter, longitudinal studies with larger sample sizes and standardized autonomic assessments are essential to confirm these results, explore their clinical relevance, and determine whether HRV markers could support risk stratification or intervention planning.
Ethical approval
The study was approved by Kocaeli University Ethics Committee (date: December 13th, 2022, number: E-80418770-020-334984).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med 2016; 375: 556-565. https://doi.org/10.1056/NEJMra1507638
- Jones GT, Herrick AL, Woodham SE, Baildam EM, Macfarlane GJ, Silman AJ. Occurrence of Raynaud’s phenomenon in children ages 12-15 years: prevalence and association with other common symptoms. Arthritis Rheum 2003; 48: 3518-3521. https://doi.org/10.1002/art.11340
- Shaffer F, Ginsberg JP. An Overview of heart rate variability metrics and norms. Front Public Health 2017; 5: 258. https://doi.org/10.3389/fpubh.2017.00258
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043-1065. https://doi.org/10.1161/01.CIR.93.5.1043
- Malpas SC, Maling TJ. Heart-rate variability and cardiac autonomic function in diabetes. Diabetes 1990; 39: 1177-1181. https://doi.org/10.2337/diab.39.10.1177
- Quintana DS, Elstad M, Kaufmann T, et al. Resting-state high-frequency heart rate variability is related to respiratory frequency in individuals with severe mental illness but not healthy controls. Sci Rep 2016; 6: 37212. https://doi.org/10.1038/srep37212
- Lindberg L, Brinth LS, Bergmann ML, et al. Autonomic nervous system activity in primary Raynaud’s phenomenon: Heart rate variability, plasma catecholamines and [123 I]MIBG heart scintigraphy. Clin Physiol Funct Imaging 2022; 42: 104-113. https://doi.org/10.1111/cpf.12737
- Karabacak K, Celik M, Kaya E, Kadan M, Arslan G, Demirkilic U. Autonomic imbalance assessed by time-domain heart rate variability indices in primary Raynaud’s phenomenon. Cardiovasc J Afr 2015; 26: 214-216. https://doi.org/10.5830/CVJA-2015-032
- Oflaz MB, Ece İ, Kibar AE, et al. Noninvasive evaluation of cardiac autonomic modulation in children with primary Raynaud’s phenomenon: a controlled study. Clin Rheumatol 2014; 33: 71-75. https://doi.org/10.1007/s10067-013-2393-1
- Maverakis E, Patel F, Kronenberg DG, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun 2014; 48-49: 60-65. https://doi.org/10.1016/j.jaut.2014.01.020
- Ingegnoli F, Zeni S, Gerloni V, Fantini F. Capillaroscopic observations in childhood rheumatic diseases and healthy controls. Clin Exp Rheumatol 2005; 23: 905-911.
- Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol 2014; 5: 1040. https://doi.org/10.3389/fpsyg.2014.01040
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5 region comparison. J Rheumatol 1997; 24: 879-889.
- Nigrovic PA, Fuhlbrigge RC, Sundel RP. Raynaud’s phenomenon in children: a retrospective review of 123 patients. Pediatrics 2003; 111: 715-721. https://doi.org/10.1542/peds.111.4.715
- Falcini F, Rigante D, Candelli M, et al. Anti-nuclear antibodies as predictor of outcome in a multi-center cohort of Italian children and adolescents with Raynaud’s phenomenon. Clin Rheumatol 2015; 34: 167-169. https://doi.org/10.1007/s10067-014-2833-6
- Pavlov-Dolijanović S, Damjanov N, Ostojić P, et al. The prognostic value of nailfold capillary changes for the development of connective tissue disease in children and adolescents with primary Raynaud phenomenon: a follow-up study of 250 patients. Pediatr Dermatol 2006; 23: 437-442. https://doi.org/10.1111/j.1525-1470.2006.00278.x
- Gasior JS, Sacha J, Jelen PJ, Pawlowski M, Werner B, Dabrowski MJ. Interaction between heart rate variability and heart rate in pediatric population. Front Physiol 2015; 6: 385. https://doi.org/10.3389/fphys.2015.00385
- Pancera P, Sansone S, Presciuttini B, et al. Autonomic nervous system dysfunction in sclerodermic and primary Raynaud’s phenomenon. Clin Sci (Lond) 1999; 96: 49-57.
- Di Franco M, Paradiso M, Riccieri V, Basili S, Mammarella A, Valesini G. Autonomic dysfunction and microvascular damage in systemic sclerosis. Clin Rheumatol 2007; 26: 1278-1283. https://doi.org/10.1007/s10067-006-0492-y
- Bertacchini P. Neurofascialvascular training for the treatment of Raynaud’s phenomenon: a case report. Mod Rheumatol Case Rep 2024; 8: 302-309. https://doi.org/10.1093/mrcr/rxae026
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















