Abstract
Background. To investigate the diagnostic and pathological staging value of serum hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606 levels in retinopathy of prematurity (ROP).
Methods. A total of 70 infants with ROP (140 eyes) treated at our hospital from October 2018 to October 2023 were enrolled as the ROP group, while 70 healthy preterm infants (140 eyes) of the same gestational age without ROP were selected as the control group. The relative expression levels of serum hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606 were detected by quantitative real-time PCR. Logistic regression analysis was used to identify factors influencing ROP occurrence. The diagnostic efficacy of the three circular RNAs (circRNAs) was evaluated using receiver operating characteristic (ROC) curve analysis.
Results. The relative expression levels of hsa_circ_0061346 in serum were significantly higher in the ROP group than in the control group (6.27 ± 3.60 vs. 0.72 ± 0.31, P < 0.05), whereas the levels of hsa_circ_0000095 (1.98 ± 1.38 vs. 3.90 ± 1.75) and hsa_circ_0068606 (1.18 ± 0.51 vs. 7.71 ± 4.45) were significantly lower (all p < 0.05). Multivariate logistic regression showed that abnormal expression of these circRNAs was an independent risk factor for ROP. Notably, the combined diagnostic performance of the three circRNAs yielded an area under the ROC curve of 0.983, with a sensitivity of 100% and a specificity of 97.14%.
Conclusion. The combined detection of serum hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606 may provide a novel approach for the early diagnosis and severity assessment of ROP.
Keywords: circular RNA, retinopathy of prematurity, biomarker
Introduction
Retinopathy of prematurity (ROP) is a leading cause of blindness in preterm infants, characterized pathologically by abnormal retinal vascular proliferation and fibrosis. With advancements in neonatal intensive care, the survival rate of preterm infants has significantly increased, but the incidence of ROP has also risen, posing serious threats to visual health and imposing substantial burdens on affected families.1-3 Currently, the diagnosis and staging of ROP primarily rely on fundus examination, which requires considerable operator expertise and cannot provide early warning signals at the molecular level. It is therefore imperative to identify biomarkers with greater sensitivity and specificity.
Recent studies have shown that circular RNAs (circRNAs) participate in the pathogenesis of various retinal diseases by regulating angiogenesis, inflammatory responses, and hypoxia-related stress.4,5 However, the role of circRNAs in ROP has not been thoroughly investigated. circTFRC (hsa_circ_0068606) has emerged as a research focus in oncology, where its aberrant expression is closely associated with tumor initiation and progression.6 Lin et al. demonstrated that hsa_circ_0068606 promotes tumor cell proliferation, migration, and invasion by regulating gene expression, acting as a miRNA sponge, and modulating signaling pathways (including ferroptosis-related pathways).7 However, its expression profile in ROP requires further investigation. Additionally, hsa_circ_0000095 and hsa_circ_0068606, located on human chromosomes 1 and 3, respectively, warrant further exploration in the context of ROP. These findings suggest that circRNAs may serve as novel molecular biomarkers for ROP, yet their expression characteristics and clinical significance in this disease remain unclear.
To our knowledge, this study is the first to systematically measure the serum expression levels of hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606 in ROP patients, analyze their associations with disease onset and pathological staging, and evaluate the clinical utility of their combined detection. The aim of this study is to explore circRNA expression profiles in ROP and identify molecules that may contribute to earlier diagnosis and improved clinical management.
Materials and Methods
Study population
A total of 70 preterm infants diagnosed with ROP (the ROP group) and 70 healthy preterm infants serving as the normal control (NC) group were enrolled from a single center (the Department of Ophthalmology and Neonatology) between October 2018 to October 2023. This study was designed and conducted as a retrospective observational study. The purpose of sample collection during 2018–2023 was for routine diagnostic evaluation, biomarker preservation, and future research aimed at improving early detection of neonatal diseases, including but not limited to ROP. Parents or legal guardians provided written informed consent for the storage and future scientific use of the biological samples and clinical data at the time of collection, regardless of whether the infants later developed ROP. The same consent process was applied to the normal control group, as their samples were likewise collected during routine clinical care and retained in accordance with institutional biobanking policies.
The sample size was estimated using the formula for comparing two independent means, with a two-sided α of 0.05 and a power of 0.80. Preliminary data from our center indicated an expected mean difference of 0.8 in circRNA expression between the ROP and NC groups, with an estimated standard deviation of 1.5. Under these assumptions, the minimum required sample size was 63 infants per group. Considering a potential 10% attrition rate, 70 infants were ultimately included in each group to ensure adequate statistical power and study reliability.
Inclusion criteria were: 1) gestational age ≤ 36 weeks; 2) birth weight ≤ 2000 g; 3) absence of congenital ocular diseases; 4) ROP diagnosis consistent with the Guidelines for Retinopathy of Prematurity Screening8; 5) informed consent obtained and ethical approval granted. Exclusion criteria included: 1) severe systemic infections or hereditary metabolic diseases; 2) a history of ocular surgery or laser treatment.
All premature infants underwent ROP screening according to our unit protocol: infants with gestational age ≤ 36 weeks or birth weight ≤ 2000 g were included, and the first examination was conducted at 4 weeks postnatal age or 35 weeks postmenstrual age (whichever came later). Subsequent examinations were performed at 1–2 weeks intervals until the retina was fully vascularized or until ROP regression or progression was determined. Screening was terminated when complete retinal vascularization was observed or when ROP had regressed without evidence of further progression.
ROP diagnosis and staging were performed via binocular indirect ophthalmoscopy after mydriasis, in accordance with the International Classification of Retinopathy of Prematurity (ICROP).8 The severity of retinal lesions was classified into Stage I (n = 4), Stage II (n = 17), Stage III (n = 42), and aggressive posterior ROP (AP-ROP, n = 7). All examinations were performed by the same senior pediatric ophthalmologist to ensure diagnostic consistency.
This study followed the principles of the Declaration of Helsinki. Ethical approval for the retrospective use of previously collected samples and clinical data was obtained from the Institutional Ethics Committee (Approval No. 2025-033). The approval covered the use of previously collected clinical data and samples collected between October 2018 and October 2023.
Sample collection and processing
Fasting venous blood (3 mL) was collected at 4 weeks postnatal age, allowed to stand at 4 °C for 30 minutes, and centrifuged at 3000 rpm (centrifugal radius: 8 cm) for 10 minutes. The supernatant serum was aliquoted into sterile microcentrifuge tubes and stored at -80 °C. Total RNA was extracted using TRIzol reagent (Invitrogen, USA), and RNA purity was assessed using a NanoDrop 2000 spectrophotometer (with an A260/A280 ratio of 1.8–2.0). The ROP stages reported in this study corresponded to the highest stage observed during follow-up, rather than the stage at the time of blood sampling.
CircRNA detection
Linear RNA was digested with RNase R (20 U/μL, Beyotime, China) at 37 °C for 30 minutes, followed by reverse transcription using HiScript III RT SuperMix (Vazyme, Nanjing, China). The quantitative reverse transcription polymerase chain reaction (qRT-PCR) reaction system (20 μL) consisted of: 10 μL SYBR Green Master Mix, 2 μL cDNA template, 1 μL each of forward and reverse primers (10 μM), and ddH2O to a total volume of 20 μL. Primer sequences are given in Table I.
| Table I. Primer sequences used in the study. | ||
| hsa_circ_0061346 | F | 5'-GAAGTGTGCCCCATTCTTTTAC-3' |
| R | 5'- TTCGCAAACATCCATCCTCT-3' | |
| hsa_circ_0000095 | F | 5'-GTATGCATACTACCTTGTACTGGTT-3' |
| R | 5'- GACTATTGAAACCTGGAGAAACT-3' | |
| hsa_circ_0068606 | F | 5'-CTGAACCAATACAGAGCAGACAT-3' |
| R | 5'- GAACTGCCACACAGAAGAACTC-3' | |
| β-actin | F | 5'-GTGACGTTGACATCCGTAAAGA-3' |
| R | 5'- GCCGGACTCATCGTACTCC-3' | |
PCR conditions were: pre-denaturation at 95°C for 5 minutes; followed by 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Relative expression levels were calculated using the 2-ΔΔCt method, normalized to β-actin as the internal reference.
Statistical analysis
Data were analyzed using SPSS 25.0 software. Categorical data were expressed as frequencies or percentages and compared using the χ² test. Normally distributed continuous data were presented as mean ± standard deviation (x ± s) and compared using the t-test for two groups or one-way analysis of variance (ANOVA) for multiple groups, with pairwise comparisons performed using the SNK-q test. To identify risk factors for ROP, univariate logistic regression analyses were first conducted. Variables with a p value <0.10 in the univariate analysis were subsequently included in the multivariate logistic regression model to calculate odds ratios (OR) and 95% confidence intervals (95% CI). The diagnostic performance was evaluated using receiver operating characteristic (ROC) curves, with the area under the curve (AUC) compared using the Z-test. For combined ROC curve analysis, the expression levels of the three circRNAs (hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606) were integrated into a logistic regression model to generate a predicted probability for each subject. These predicted probabilities were then used to construct a combined ROC curve, allowing assessment of the overall diagnostic performance of the three markers together. A p-value <0.05 was considered statistically significant.
Results
Comparison of clinical characteristics between ROP and control groups
A total of 70 preterm infants with retinopathy of prematurity (the ROP group) and 70 healthy preterm infants (NC group) were included. The mean gestational age of infants in the ROP group was 30.57±3.16 weeks, which was significantly lower than that of the NC group (34.97 ± 1.74 weeks, p < 0.05). Among the 70 ROP patients, 23 infants (32.9%) received treatment, while the remaining 47 infants (67.1%) experienced spontaneous regression of ROP (i.e., the disease resolved without intervention). No significant differences were observed between the two groups in terms of sex, singleton/twin status, gestational diabetes, maternal infection, gestational hypertension, bronchopulmonary dysplasia (BPD), or 1-minute Apgar scores (p > 0.05). However, the birth weight in the ROP group (1.56 ± 0.54 kg) was significantly lower than that in the NC group (2.38 ± 0.55 kg) (p < 0.001) (Table II, Fig. 1A). Additionally, the proportion of infants with neonatal respiratory distress syndrome (NRDS) was significantly higher in the ROP group compared to the NC group (χ² = 8.96, p = 0.0028).
|
Percentages are column percentages. BPD: Bronchopulmonary dysplasia, NRDS: Neonatal respiratory distress syndrome. |
||||
| Table II. Comparison of clinical characteristics between ROP and control groups [n (%) or x±s] | ||||
| Clinical parameter |
|
|
|
|
| Gestational age (weeks) |
|
|
|
|
| Birth weight (kg) |
|
|
|
|
| Male sex |
|
|
|
|
| Twin birth |
|
|
|
|
| Gestational diabetes |
|
|
|
|
| Maternal infection |
|
|
|
|
| Gestational hypertension |
|
|
|
|
| BPD |
|
|
|
|
| NRDS |
|
|
|
|
| Apgar score < 8 (1 min) |
|
|
|
|
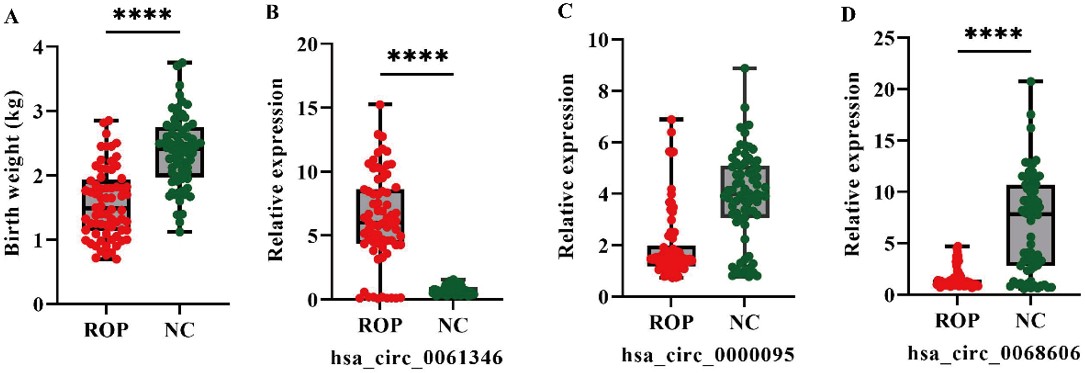
ROP: retinopathy of prematurity, NC: normal control.
Comparison of serum circRNA expression levels between groups
Serum expression of hsa_circ_0061346 was significantly higher in the ROP group (6.27 ± 3.60) compared to the NC group (0.72 ± 0.31). In contrast, expression levels of hsa_circ_0000095 (1.98 ± 1.38 vs. 3.90 ± 1.75) and hsa_circ_0068606 (1.18 ± 0.51 vs. 7.71 ± 4.45) were significantly lower in the ROP group compared to the NC group (Fig. 1B-D).
Changes in circRNA levels across different clinical characteristics in ROP
The results in Table III indicate variations in the expression levels of the three circRNAs across different clinical characteristics. The expression of hsa_circ_0061346 was lower in the maternal infection group (5.47 ± 3.31) compared to the non-infection group (7.12 ± 3.74). The expression of hsa_circ_0000095 was significantly higher in the gestational diabetes group (1.79 ± 1.14) compared to the non-diabetes group (1.33 ± 0.77, p = 0.046). Notably, hsa_circ_0068606 expression was significantly higher in the BPD group (3.04 ± 2.17) compared to the non-BPD group (1.87 ± 1.24, p = 0.03) and in the 1-minute Apgar score < 8 group (2.97 ± 0.04) compared to the ≥ 8 group (1.88 ± 1.27, p = 0.047). No statistically significant differences were observed in the expression levels of the three circRNAs across sex, singleton/twin status, gestational hypertension, NRDS, or different pathological stages (p > 0.05).
|
Data are presented as mean ± standard deviation (SD). BPD, Bronchopulmonary dysplasia; NRDS, Neonatal respiratory distress syndrome; ROP, retinopathy of prematurity; AP-ROP, aggressive posterior ROP. |
|||
| Table III. Comparison of three circRNAs with clinical characteristics in the ROP group | |||
| Clinical parameter |
|
|
|
| Sex |
|
|
|
| Male |
|
|
|
| Female |
|
|
|
| Singleton/twin |
|
|
|
| Singleton |
|
|
|
| Twin |
|
|
|
| Gestational diabetes |
|
|
|
| No |
|
|
|
| Yes |
|
|
|
| Maternal infection |
|
|
|
| No |
|
|
|
| Yes |
|
|
|
| Gestational hypertension |
|
|
|
| No |
|
|
|
| Yes |
|
|
|
| BPD |
|
|
|
| No |
|
|
|
| Yes |
|
|
|
| NRDS |
|
|
|
| No |
|
|
|
| Yes |
|
|
|
| 1 min Apgar score |
|
|
|
| ≥8 |
|
|
|
| <8 |
|
|
|
| ROP stage |
|
|
|
| I |
|
|
|
| II |
|
|
|
| III |
|
|
|
| AP-ROP |
|
|
|
Multivariate logistic regression analysis
Univariate analysis (Table IV) revealed that gestational age (β = -0.641, OR = 0.527, p < 0.0001), birth weight (β = -2.493, OR = 0.083, p < 0.0001), and NRDS (β = 1.170, OR = 3.222, p = 0.004) were significantly associated with ROP occurrence. Lower gestational age and lower birth weight were associated with a higher risk of ROP, while NRDS was also associated with an increased risk. Notably, all three circRNA molecules (hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606) showed significant statistical associations (p < 0.0001 for all), with hsa_circ_0061346 expression associated with a higher risk of ROP (OR = 3.240), and higher expression of hsa_circ_0000095 and hsa_circ_0068606 associated with a lower risk (OR = 0.483 and 0.507, respectively). Other clinical factors, such as sex, singleton/twin status, and gestational complications, showed no significant associations.
| BPD, Bronchopulmonary dysplasia; NRDS, Neonatal respiratory distress syndrome; ROP, retinopathy of prematurity; SE, Standard error; OR, Odds Ratio. | ||||||
| Table IV. Univariate logistic regression analysis of factors associated with ROP occurrence | ||||||
| Factor |
|
|
|
|
|
|
| Sex |
|
|
|
|
|
|
| Gestational age |
|
|
|
|
|
|
| Birth weight |
|
|
|
|
|
|
| Singleton/twin |
|
|
|
|
|
|
| Gestational diabetes |
|
|
|
|
|
|
| Maternal infection |
|
|
|
|
|
|
| Gestational hypertension |
|
|
|
|
|
|
| BPD |
|
|
|
|
|
|
| NRDS |
|
|
|
|
|
|
| 1 min Apgar score |
|
|
|
|
|
|
| hsa_circ_0061346 |
|
|
|
|
|
|
| hsa_circ_0000095 |
|
|
|
|
|
|
| hsa_circ_0068606 |
|
|
|
|
|
|
Multivariate logistic regression analysis (Table V) indicated that, after adjusting for other variables, hsa_circ_0061346 (β = 1.195, OR = 3.303, 95% CI: 2.183–6.535, p < 0.0001) remained significantly associated with an increased risk of ROP, while hsa_circ_0000095 (β = -0.978, OR = 0.376, 95% CI: 0.188–0.574, p = 0.0005) and hsa_circ_0068606 (β = -1.291, OR = 0.275, 95% CI: 0.147–0.435, p< 0.0001) retained significant protective effects. Notably, gestational age (p = 0.851) and birth weight (p = 0.089) were no longer statistically significant in the multivariate model, and the effect of NRDS was attenuated (p = 0.190).
| BPD, Bronchopulmonary dysplasia; NRDS, Neonatal respiratory distress syndrome; ROP, retinopathy of prematurity; SE, Standard error; OR, Odds Ratio. | ||||||
| Table V. Multivariate logistic regression analysis of factors associated with ROP occurrence | ||||||
| Factor |
|
|
|
|
|
|
| Gestational age |
|
|
|
|
|
|
| Birth weight |
|
|
|
|
|
|
| NRDS |
|
|
|
|
|
|
| hsa_circ_0061346 |
|
|
|
|
|
|
| hsa_circ_0000095 |
|
|
|
|
|
|
| hsa_circ_0068606 |
|
|
|
|
|
|
ROC curve analysis
The AUC for the individual diagnosis of ROP using serum hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606 was 0.862 (95% CI: 0.782–0.942) (Fig. 2A), 0.778 (95% CI: 0.693–0.862) (Fig. 2B), and 0.826 (95% CI: 0.748–0.903) (Fig. 2C), respectively. The combined diagnosis using all three circRNAs yielded an improved AUC of 0.983 (95% CI: 0.937–0.998) (Fig. 2D), with a sensitivity of 100% and a specificity of 97.14%. Z-test results indicated that the diagnostic performance of the combined approach was significantly superior to that of individual markers (Z = -20.72 to -3.366, all p < 0.001).
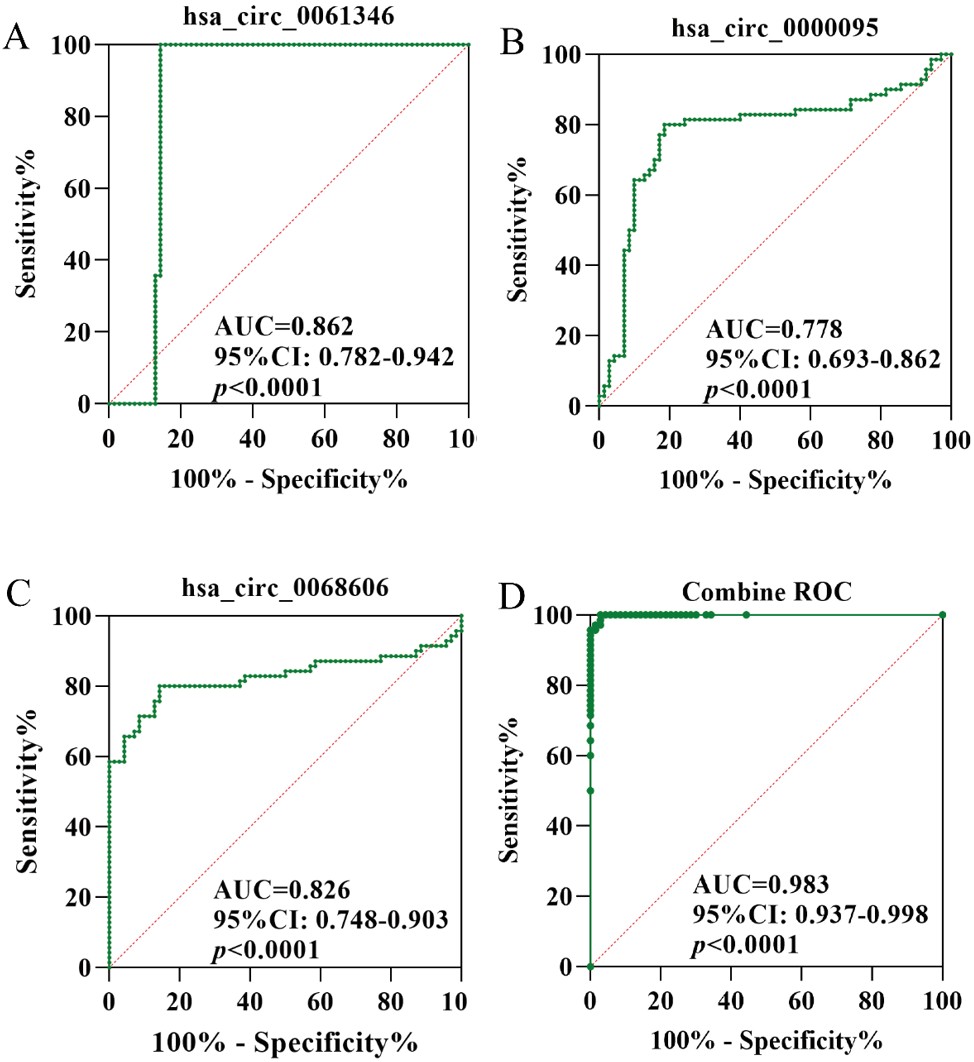
ROC: receiver operating characteristic, ROP: retinopathy of prematurity.
Discussion
ROP is a leading cause of childhood blindness and its pathogenesis has not yet been fully elucidated. CircRNAs, as a novel subtype of non-coding RNAs, have emerged as promising biomarkers and potential therapeutic targets in various ophthalmic diseases.9,10 This study represents the first systematic evaluation of the expression profiles and clinical diagnostic utility of serum hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606 in ROP. Our findings demonstrate that these three circRNAs exhibit aberrant expression in ROP patients, with significant diagnostic and predictive value. Notably, their combined detection achieved an AUC of 0.983, with high sensitivity and specificity, highlighting their substantial potential as molecular biomarkers for early ROP detection. Specifically, serum hsa_circ_0061346 expression was significantly elevated in the ROP group and showed a significant association with an increased risk of ROP occurrence (OR=3.303, p < 0.0001). Recent studies have indicated that circRNAs play a critical regulatory role in retinal vascular diseases.11,12 Li et al. reported the role of altered circRNA expression in peripheral blood mononuclear cells in retinal disorders13, which supports the observations of this study.
In contrast, hsa_circ_0000095 and hsa_circ_0068606 exhibited significantly reduced expression in the ROP group and were identified as protective factors, with OR values of 0.376 and 0.275, respectively. These findings suggest that these circRNAs may contribute to suppressing abnormal vascular proliferation associated with ROP or to promoting retinal vascular stability, potentially through competitive binding with miRNAs or the regulation of signaling pathways. hsa_circ_0068606 has been extensively studied in tumor biology, where it promotes cell proliferation and migration by regulating ferroptosis and MAPK-related signaling pathways.7 Given that ROP pathogenesis involves hypoxia, iron metabolism dysregulation, and oxidative stress.14-16 the reduced expression of hsa_circ_0068606 observed in this study may indicate a loss of its inhibitory effect on these aberrant signals, thereby promoting ROP progression.
Additionally, the associations between the three circRNAs and specific clinical characteristics suggest differential regulatory patterns under various pathological conditions. For instance, hsa_circ_0000095 expression was elevated in infants with gestational diabetes, while hsa_circ_0068606 levels were higher in those with BPD and lower 1-minute Apgar scores, potentially reflecting adaptive changes in circRNA expression across distinct pathological contexts, which warrants further investigation. Multivariate logistic regression analysis further supported the independent diagnostic value of circRNAs in ROP. After adjusting for perinatal risk factors, the significance of the three circRNAs persisted, whereas conventional indicators such as gestational age and birth weight were no longer statistically significant in the multivariate model. This finding underscores the central role of circRNAs in disease mechanisms and highlights the limitations of current clinical assessment systems, particularly in early disease stages, where molecular biomarkers may outperform traditional clinical characteristics.
However, this study has several limitations. First, the sample size was relatively small, and although intergroup matching was well-controlled, the generalizability and stability of the results needs further validation in multicenter studies with larger cohorts. Second, there was a significant difference in gestational age between the ROP and NC groups, which may have influenced the levels of the circRNAs examined and introduced a potential confounding factor in the interpretation of the findings. Third, the functional mechanisms of circRNAs were not thoroughly validated in vitro or in animal models. Future studies should incorporate functional experiments (e.g., miRNA binding and target gene regulation analyses) to elucidate their biological roles. Additionally, while qRT-PCR is a sensitive detection method, its routine clinical application faces challenges related to standardization and operational complexity.
In conclusion, this study demonstrates that hsa_circ_0061346, hsa_circ_0000095, and hsa_circ_0068606 exhibit significantly aberrant expression in the serum of ROP patients and are strongly associated with disease occurrence. Their combined detection substantially enhances diagnostic performance. As stable and specific molecular biomarkers, circRNAs hold promise as novel tools for early ROP screening and staging. Furthermore, these circRNAs may play a critical role in ROP pathogenesis, providing new insights for future mechanistic studies and the development of targeted interventions.
Ethical approval
The study was approved by The Huadu District Maternal and Child Health Hospital (number: 2025-033).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Sabri K, Ells AL, Lee EY, Dutta S, Vinekar A. Retinopathy of prematurity: a global perspective and recent developments. Pediatrics 2022; 150: e2021053924. https://doi.org/10.1542/peds.2021-053924
- Hong EH, Shin YU, Cho H. Retinopathy of prematurity: a review of epidemiology and current treatment strategies. Clin Exp Pediatr 2022; 65: 115-126. https://doi.org/10.3345/cep.2021.00773
- Gebeşçe A, Uslu H, Keleş E, et al. Retinopathy of prematurity: incidence, risk factors, and evaluation of screening criteria. Turk J Med Sci 2016; 46: 315-320. https://doi.org/10.3906/sag-1407-127
- Kim H, Kim J, Ryu J. Noncoding RNAs as a novel approach to target retinopathy of prematurity. Front Pharmacol 2022; 13: 1033341. https://doi.org/10.3389/fphar.2022.1033341
- Zhou H, Song H, Wu Y, et al. Oxygen-induced circRNA profiles and coregulatory networks in a retinopathy of prematurity mouse model. Exp Ther Med 2019; 18: 2037-2050. https://doi.org/10.3892/etm.2019.7819
- Yan Z, Duan C, Li X, et al. circ-TFRC downregulation suppresses ovarian cancer progression via miR-615-3p/IGF2 axis regulation. Cancer Cell Int 2024; 24: 152. https://doi.org/10.1186/s12935-024-03287-4
- Lin Z, Zhong C, Shi M, et al. Circular RNA TFRC/SCD1 mRNA interaction regulates ferroptosis and metastasis in gastric cancer. Cell Death Dis 2025; 16: 436. https://doi.org/10.1038/s41419-025-07759-x
- Wilkinson AR, Adams GGW, Fleck BW, Nieto-Hernandez R; Guideline Development Groups (GDG) of the Royal College of Paediatrics and Child Health (RCPCH) and the Royal College of Ophthalmologists (RCOphth). UK screening and treatment of retinopathy of prematurity updated 2022 guidelines. Early Hum Dev 2023; 177-178: 105715. https://doi.org/10.1016/j.earlhumdev.2023.105715
- Hanineva A, Park KS, Wang JJ, DeAngelis MM, Farkas MH, Zhang SX. Emerging roles of circular RNAs in retinal diseases. Neural Regen Res 2022; 17: 1875-1880. https://doi.org/10.4103/1673-5374.335691
- Zhang C, Hu J, Yu Y. CircRNA is a rising star in researches of ocular diseases. Front Cell Dev Biol 2020; 8: 850. https://doi.org/10.3389/fcell.2020.00850
- Wu W, Zhang Y, Yang M. Emerging role of circular RNAs in the pathogenesis of retinoblastoma. Ophthalmic Res 2024; 67: 51-61. https://doi.org/10.1159/000535329
- Sun LF, Chen XJ, Jin ZB. Emerging roles of non-coding RNAs in retinal diseases: a review. Clin Exp Ophthalmol 2020; 48: 1085-1101. https://doi.org/10.1111/ceo.13806
- Li Y, Zhou H, Huang Q, et al. Potential biomarkers for retinopathy of prematurity identified by circular RNA profiling in peripheral blood mononuclear cells. Front Immunol 2022; 13: 953812. https://doi.org/10.3389/fimmu.2022.953812
- Zhang L, Buonfiglio F, Fieß A, Pfeiffer N, Gericke A. Retinopathy of prematurity-targeting hypoxic and redox signaling pathways. Antioxidants (Basel) 2024; 13: 148. https://doi.org/10.3390/antiox13020148
- Liu CQ, Liu XY, Ouyang PW, et al. Ferrostatin-1 attenuates pathological angiogenesis in oxygen-induced retinopathy via inhibition of ferroptosis. Exp Eye Res 2023; 226: 109347. https://doi.org/10.1016/j.exer.2022.109347
- Graziosi A, Perrotta M, Russo D, et al. Oxidative stress markers and the retinopathy of prematurity. J Clin Med 2020; 9: 2711. https://doi.org/10.3390/jcm9092711
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















