Abstract
Background. Gasdermin-D (GSDMD) is an inflammasome regulator. Pyroptosis and GSDMD-mediated interleukin (IL)-1β secretion abolish in GSDMD-deficient familial Mediterranean fever (FMF) knock-in mice. We aimed to investigate GSDMD gene expression (GSDMD-∆), acute phase reactants (APRs), serum IL-1β, and IL-18 levels in FMF patients during attacks and attack-free periods.
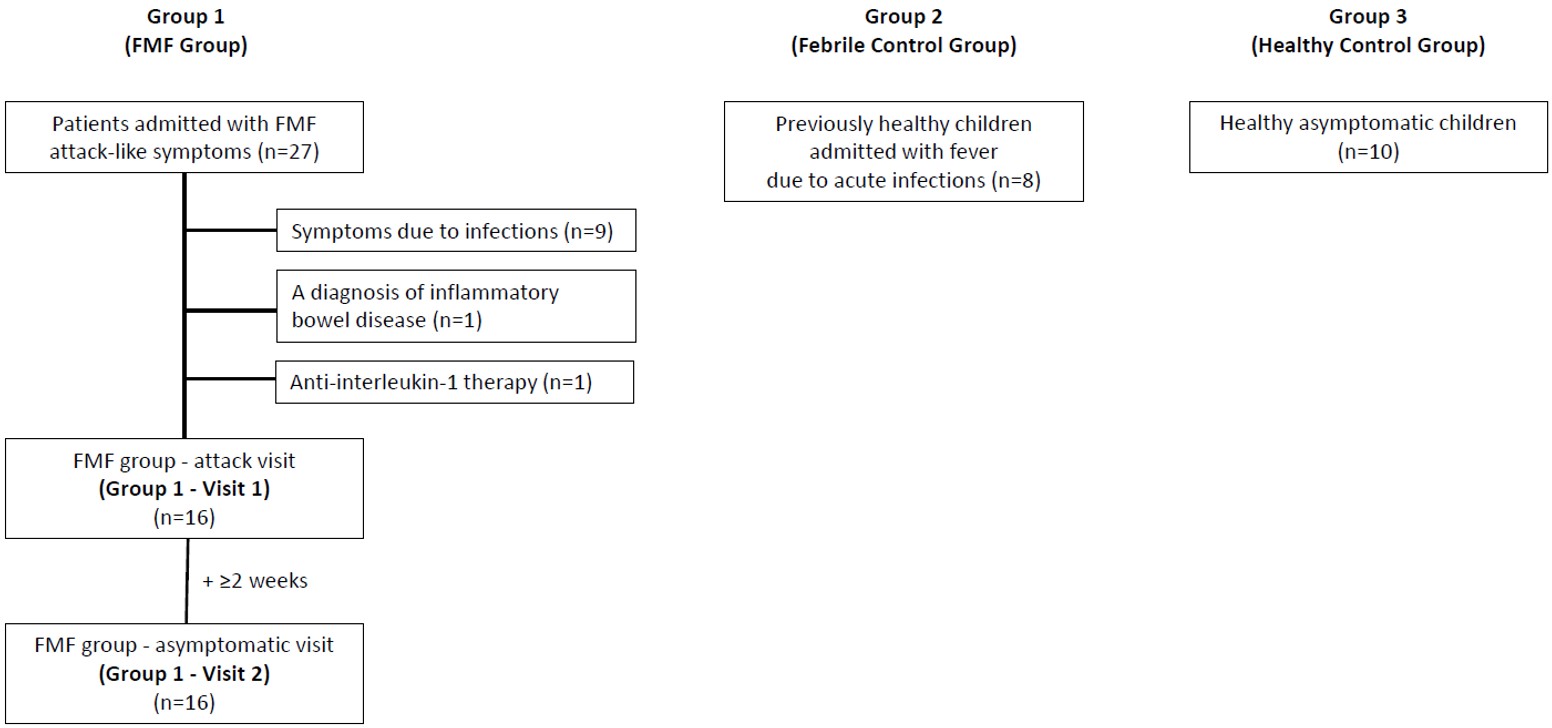
Methods. We tested GSDMD-∆, serum APRs, and serum IL-1β and IL-18 in 16 FMF patients (G1), during attack (G1-V1) and at attack-free visits (G1-V2). The GSDMD-∆, serum IL-1β and IL-18 were measured in febrile controls with acute infections (G2) and healthy children (G3).
Results. Age and sex distribution of patients and controls were similar. Median GSDMD-∆ was 10 times higher in G1-V1 compared to G1-V2 (p<0.001). GSDMD-∆ was four times higher in G1-V1 than those observed in G2 (p=0.026); however, serum APRs were similar between these groups. GSDMD-∆ in G1-V2 and G3 did not differ significantly (p>0.05). GSDMD-∆ in G1 strongly correlated with serum C-reactive protein and amyloid-A (r>0.60, p<0.01) but did not correlate with serum IL-1β and IL-18. Median GSDMD-∆ and serum APRs were similar in patients carrying biallelic and monoallelic ‘exon 10’ mutations in MEFV gene both during attacks and attack-free visits (p>0.05).
Conclusion. We showed a significantly increased GSDMD-∆ for the first time in humans, thereby indicating the distinct role of GSDMD-∆ as a biomarker similar to APRs in FMF attacks. It was even higher than levels detected during acute infections, supporting the functional involvement of GSDMD-∆ in FMF attacks. GSDMD-∆ correlated with APRs but not with serum IL-1β and IL-18 levels.
Keywords: Familial Mediterranean fever, gasdermins, MEFV, infection
Introduction
Systemic autoinflammatory diseases (AIDs) are characterized by recurrent, spontaneous, and inflammatory febrile attacks, primarily driven by dysregulation in the innate immune system.1 There has been a shift from a gene-centric perspective to a systems-based classification to understand the intricate molecular mechanisms underlying AIDs.2 Gasdermin-D (GSDMD) functions as a critical molecule in inflammasome response. Its activation through autoinhibition requires the cleavage of its N-terminal domain by caspase-1. Upon activation, GSDMD mediates the secretion of activated proinflammatory cytokines and intracellular components to the extracellular area by forming pores in the cell membrane, thus contributing to inflammasome-related pyroptosis.3 Additionally, GSDMD plays a crucial role in neutrophil-extracellular trap generation and release, which further amplifies the inflammatory response.4 Besides, GSDMD functions as a feedback regulator for inflammasome activation, highlighting its significance in various inflammatory, infectious, and autoimmune disease mechanisms and presenting it as a promising therapeutic target.5
Familial Mediterranean fever (FMF), classified under pyrin-related AIDs, is the most common AID.6 The gain-of-function (GOF) mutations in the Mediterranean Fever (MEFV) gene encoding pyrin lead to a lowered activation threshold of the pyrin inflammasome, resulting in inflammatory manifestations characteristic of FMF.7 The pyrin-caspase-1-GSDMD pathway is the central pathway of autoinflammation in FMF. The release of interleukin (IL)-1β, IL-18, and alarmin molecules is related to GSDMD through pyrin and caspase-1 activation.8,9
Typical inflammatory manifestations of FMF include non-periodic recurrent fever, serositis, and synovitis, which last for 6-72 hours, accompanied by increased serum acute phase reactants (APRs).10 While there remains a lack of a clear genotype-phenotype correlation, it is noteworthy that biallelic ‘exon 10’ mutations in the MEFV gene are associated with higher disease activity, earlier disease onset, and renal amyloidosis compared to monoallelic mutations or mutations in non-‘exon 10` regions.11-15 Furthermore, pyrin inflammasome response defined by the magnitude of IL-1β secretion has been found to be higher in patients bearing biallelic `exon 10` mutations compared to those with a monoallelic `exon 10` mutations when stimulated by a bacterial toxin specifically activating the pyrin inflammasome.7
The primary objective of this study was to investigate the changes in GSDMD during FMF attacks compared with attack-free periods and healthy children with and without acute infections, in relation to serum APRs and serum IL-1β and IL-18. Additionally, we aimed to explore whether biallelic or monoallelic `exon 10` mutations in the MEFV gene exhibited a gene dosage effect on GSDMD gene expression (GSDMD-∆).
Materials and Methods
Study design
Patients diagnosed with FMF according to Turkish pediatric FMF criteria were eligible for the study.16 The MEFV gene mutations were examined through next-generation sequencing (NGS, QIAseq Targeted DNA FMF kit, Qiagen, Germany), and we further used an expanded periodic fever syndrome/AID NGS panel for a patient with a nonconfirmatory genotype of FMF.17 The pathogenicity of the genetic variants in the MEFV gene was classified.18 Patient recruitment and sample collection were completed between October 2022 and May 2023.
For the study, we evaluated FMF patients (Group 1 [G1], n=16) admitted during a disease attack (Group 1-Visit 1 [G1-V1]) and during a symptom-free period at least two weeks following the resolution of the disease attack (Group 1-Visit 2 [G1-V2]). We excluded FMF patients presenting with attack-like symptoms originating from other etiologies, such as infections and FMF-associated diseases, and those treated with biologics. We noted the compliance with colchicine for patients with a previous diagnosis of FMF. We grouped the FMF patients further according to the MEFV gene mutations (biallelic and monoallelic ‘exon 10’ mutations).
The febrile control group and healthy control group were recruited as age- and sex-matched to the FMF group. Healthy children without any chronic disease were enrolled during a febrile acute infectious disease and classified as the febrile control group (Group 2 [G2], n=8) and healthy children without symptoms as the healthy control group (Group 3 [G3], n=10). The duration of fever was noted for the FMF group-attack visit (G1-V1) and febrile control group (G2) and their blood samples were collected after at least 8 hours but before 48 hours of fever. Children with a history of AIDs, both in themselves or their families, were excluded from both control groups. The flow diagram of patient recruitment is presented in Fig. 1.
We recorded the demographic data, clinical/laboratory findings, and current medications from electronic patient medical files and evaluated the weight and height of the patients according to the Turkish children’s growth standards.19 For the FMF group, the disease severity was calculated by the International Severity Scoring System for FMF (ISSF) which was validated for use in children and adults with FMF. A score of 3 or higher represents moderate-to-severe disease.20
Complete blood cell count with differential was tested in all study participants. We obtained serum APRs (C-reactive protein (CRP, N <5 mg/L), amyloid-A (SAA, N <0.5 mg/dL), fibrinogen (N <4.2 g/L), and erythrocyte sedimentation rate (ESR, N<15 mm/h)) for the FMF group at two visits (G1-V1 and G1-V2) and for the febrile control group (G2). We measured serum IL-1β and IL-18 levels with enzyme-linked immunosorbent assay (ELISA) and GSDMD-∆ by real-time polymerase chain reaction (RT-PCR).
We obtained written informed consent from all participants. The study complied with the Declaration of Helsinki, and the Clinical Research Ethics Committee of Hacettepe University Faculty of Medicine approved the study protocol. This work was supported by the Scientific Research Projects (BAP) Coordination Unit of Hacettepe University.
Measurement of serum IL-1β and IL-18
Serum was extracted from the peripheral blood by centrifugation at 3500 g for 15 minutes at +4 °C, subsequently transferred to sterile tubes and stored at -20°C. The tubes were stored at -80 °C if the laboratory analysis was planned for later than three months.
Serum IL-1β and IL-18 were analyzed using human IL-1β and IL-18 sandwich ELISA kits (BT Lab, Shanghai Korain Biotech Co., Ltd., China). We used the manufacturer’s instructions and tested all samples twice. We measured the optical density (OD) spectrophotometrically at 450 nm in a microplate reader (SPECTROstar Nano, BMG LABTECH GmbH, Germany). Serum IL-1β (N <12 pg/mL) and IL-18 (N <120 pg/mL) levels were compared and quantified with the standard curves as pg/mL.
Measurement of GSDMD gene expression
We collected the peripheral whole blood into the PAXgene Blood RiboNucleic Acid (RNA) Tubes (Qiagen, Germany). The tubes were gently inverted, and stored at room temperature for 2-6 hours and then moved to +4°C for 1-2 days. Subsequently, we stored the tubes at -20°C if we planned to do the RT-PCR for less than three months. The samples were transferred to -80°C if we planned to perform RT-PCR for later than three months. We used a reference gene (glyceraldehyde-3-phosphate dehydrogenase, GADPH) to normalize GSDMD-∆ levels.
Ribonucleic acid was extracted according to the manufacturer’s protocol from the peripheral blood mononuclear cells using the PAX gene Blood RNA System Kit. We measured the extracted deoxyribonucleic acid (DNA) using a NanoDrop ND-1000 spectrophotometer (Labtech International, UK). The average RNA concentration was 173.1 ng/μL. The quality output was evaluated with 260/280 and 260/230 ratios of purity estimations.
We used Quantitech Reverse Transcription Kit (Qiagen, Germany) for ribonucleic acid reverse transcription following the manufacturer`s protocol. We used specific primers and TaqMan probes for the target and housekeeping genes. Amplification occurred on the RT-PCR Corbett Rotor-Gene 6000. We tested each sample twice.
The threshold value was 10-3, and the cycle threshold value (Ct) was 40 cycles. To evaluate the GSDMD-∆ level in the FMF and febrile control groups, we used the median fold change of GSDMD-∆ in the healthy control group. We performed the delta-delta Ct method to analyze RT-PCR data as a relative quantification method.
Statistical analysis
IBM SPSS Statistics (SPSS v21.0, IBM Corp, NY, USA) was used for statistical analysis. Descriptive statistics were presented with continuous variables with mean ± standard deviation (SD) or median (minimum-maximum) and categorical variables with frequency (n (%)). The distribution of variables was evaluated by distribution graphs and the Shapiro-Wilk test. One-way ANOVA was used for parametric data and the Mann-Whitney U test for nonparametric data. Fisher’s exact test was used to analyze differences between categorical variables. Spearman’s rho correlation test analyzed the correlation between clinical and laboratory data. The correlation coefficient (r) classified correlation significance as weak if r=0.2-0.4, moderate if r=0.4-0.6, and strong if r≥0.6.21 The statistical significance level was accepted as p<0.05.
Results
Study participants
Sixteen patients with FMF (mean age 8 years and 56% female) evaluated both during a disease attack (G1-V1) and an attack-free visit (G1-V2) were recruited to the study. Demographic, clinical, and laboratory findings of the patients with FMF are presented in Table I. The mean age at the diagnosis of FMF was 3.3 ± 2.2 years. Parental consanguinity was present in 37.5% of the patients. The mean standard deviation scores of weight and height were within normal ranges (0.2 ± 1.0 and 0.6 ± 1.0, respectively).
| *Normal ranges for serum acute phase reactants: CRP <5 mg/L; SAA <0.5 mg/dL; fibrinogen <4.2 g/L; ESR <15 mm/h. CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; ISSF: International Severity Scoring System for Familial Mediterranean Fever; SAA: serum amyloid-A; SD: Standard Deviation. | |
| Table I. Demographic, clinical, and laboratory findings of patients with familial Mediterranean fever. | |
| Demographic, clinical, and laboratory findings | Results (n=16) |
| Age in years, mean ± SD | 8.0 ± 3.8 |
| Female:Male | 9:7 |
| Consanguinity, n (%) | 6 (37.5) |
| Family history, n (%) | |
| Familial Mediterranean fever | 8 (50.0) |
| Secondary amyloidosis | 1 (6.3) |
| Recurrent clinical findings of familial Mediterranean fever, n (%) | |
| Fever | 16 (100) |
| Abdominal pain | 13 (81.3) |
| Chest pain | 4 (25.0) |
| Arthritis | 1 (6.3) |
| Erysipelas-like erythema | 1 (6.3) |
| Total ISSF score, mean ± SD | 3.0 ± 1.8 |
| Serum acute phase reactants, median (min-max)* | |
| Attack visit | |
| CRP, mg/L | 45.1 (9.1-108.0) |
| SAA, mg/dL | 44.1 (2.9-127.0) |
| Fibrinogen, g/L | 3.9 (2.4-5.8) |
| ESR, mm/h | 25.0 (7.0-43.0) |
| Attack-free visit | |
| CRP, mg/L | 1.8 (0.4-6.6) |
| SAA, mg/dL | 0.4 (0.2-7.9) |
| Fibrinogen, g/L | 2.6 (2.0-3.8) |
| ESR, mm/h | 10.5 (4.0-41.0) |
The most common clinical findings of FMF were fever (100%) and abdominal pain (81.3%). Disease severity, as defined by the ISSF score, was categorized as moderate-to-severe in 50% of the patients while the remaining had mild disease. Eleven patients had a new diagnosis of FMF and two patients with a prior diagnosis were noncompliant with colchicine.
Half of the FMF group (n=8) had biallelic ‘exon 10’ mutations in the MEFV gene, whereas seven patients had monoallelic ‘exon 10’ mutation either alone (n=5) or compound heterozygous with an ‘exon 2’ (E148Q) mutation (n=2) (Supplementary Table S1). One patient had heterozygous `exon 2` mutation (E148Q/-) in the MEFV gene. The expanded periodic fever syndrome panel resulted negative in this patient who was excluded from the group comparisons. The clinical and laboratory findings did not differ in FMF patients carrying biallelic and monoallelic `exon 10` mutations in the MEFV gene (Supplementary Table S2).
The febrile control group (G2, n=8) was found to have acute upper respiratory tract infection (n=5), gastroenteritis (n=2), and lower respiratory tract infection (n=1). Microbial cultures revealed Group A beta-hemolytic streptococcus in two patients with upper and Streptococcus pneumoniae in one patient with lower respiratory tract infection. The mean duration of fever at blood collection was 28.5 ± 16.5 hours for the febrile control group (G2), whereas it was 22.6 ± 12.7 hours for the FMF group-attack visit (G1-V1), and it was not statistically different between G1-V1 and G2 (p=0.343). The maximum duration of fever at blood drawn was 48 hours in both groups.
Serum acute phase reactants, serum IL-1β and IL-18, and GSDMD gene expression
Table II shows the comparison of serum APRs, serum IL-1β and IL-18, and GSDMD-∆ levels between the groups (G1-V1, G1-V2, G2, G3). All patients with FMF had significantly increased serum APRs during the attack visits compared to their levels at attack-free visits (all p≤0.005). The most significant increase was observed in SAA (39.0 [2.9-127.0] vs. 0.5 [0.2-7.9] mg/dL, p<0.001) and CRP (41.2 [9.1-99.6] vs. 1.9 [0.6-6.6] mg/L, p<0.001). The comparison of the FMF group-attack visit (G1-V1) with the febrile control group (G2) demonstrated increased APRs without statistical significance (all p>0.05).
| Normal ranges for serum acute phase reactants and interleukins: CRP <5 mg/L; SAA <0.5 mg/dL; fibrinogen <4.2 g/L; ESR <15 mm/h; IL-1β <12 pg/mL; IL-18 <120 pg/mL. CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; G1-V1: FMF group-attack visit; G1-V2: FMF group-attack-free visit; G2: febrile control group; G3: healthy control group; GSDMD: gasdermin-D; GSDMD-∆: gasdermin-D gene expression; NA: not applicable; SAA: serum amyloid-A. *Median (min-max); **mean 2^(-delta delta Ct); #p-value. | ||||||||||
| Table II. Comparison of serum acute phase reactants, serum IL-1β and IL-18, and GSDMD gene expression in groups*. | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| CRP, mg/L |
|
|
|
|
|
|
|
|
|
|
| SAA, mg/dL |
|
|
|
|
|
|
|
|
|
|
| Fibrinogen, g/L |
|
|
|
|
|
|
|
|
|
|
| ESR, mm/h |
|
|
|
|
|
|
|
|
|
|
| IL-1β, pg/mL |
|
|
|
|
|
|
|
|
|
|
| IL-18, pg/mL |
|
|
|
|
|
|
|
|
|
|
| GSDMD-∆** |
|
|
|
|
|
|
|
|
|
|
The median of serum IL-1β and IL-18 were measured within normal ranges at both visits of the FMF group (G1-V1 vs. G1-V2) without statistical significance. We found that `G1-V1 vs. G3`, `G1-V2 vs. G3`, and `G2 vs. G3` differed in terms of the serum IL-1β and IL-18 where G1 and G2 had significantly higher levels compared to G3.
The distribution of GSDMD-∆ across study groups is provided in Fig. 2. `G1V1 and G3` differed significantly in terms of GSDMD-∆ levels (p<0.001), whereas GSDMD-∆ levels were similar in G1-V2, G2, and G3. The median GSDMD-∆ increased more than 10 times in the FMF group-attack visit (G1-V1) compared to the attack-free visit (G1-V2) (p<0.001). On the other hand, when compared to the febrile control group (G2), a more than four times significant increase in GSDMD-∆ levels were detected in the FMF group-attack visit (G1-V1) (p=0.026).
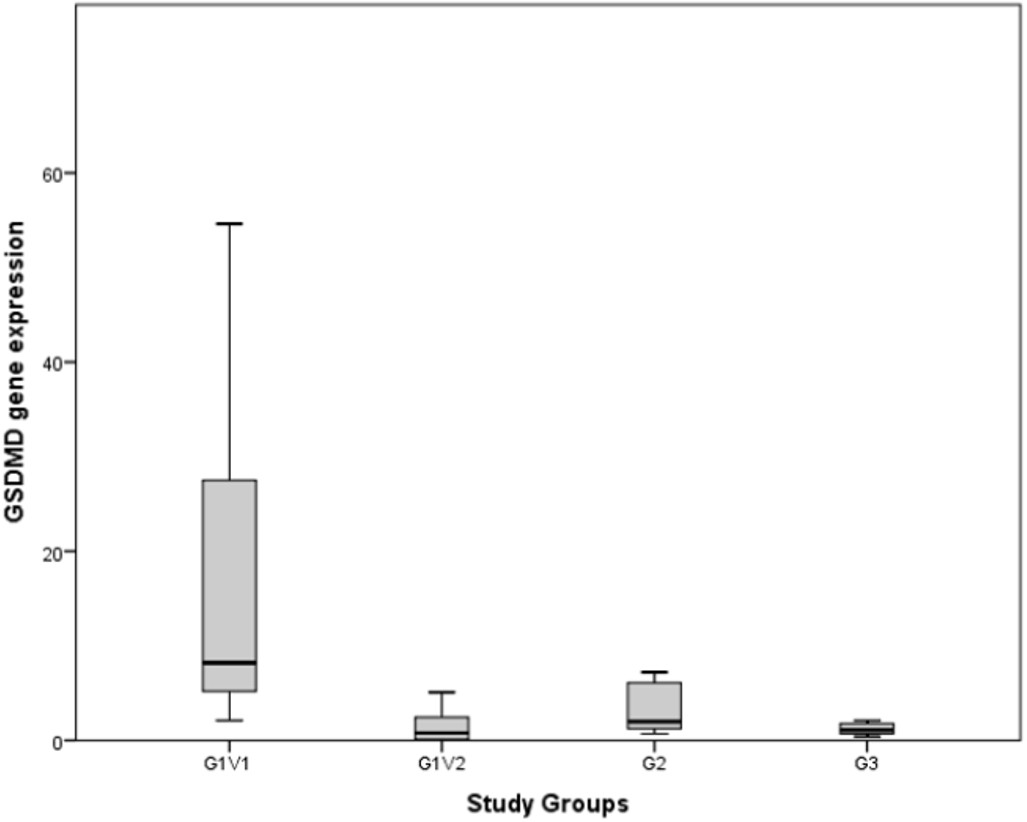
G1-V1: FMF group-attack visit, G1-V2: FMF group-attack-free visit, G2: febrile control group, G3: healthy control group.
Correlation between serum acute phase reactants, serum IL-1β and IL-18, and GSDMD gene expression
The correlation analysis between serum APRs, serum IL-1β and IL-18, and GSDMD-∆ is shown in Table III for all participants and the FMF group, separately. The GSDMD-∆ demonstrated a moderate correlation with serum CRP, SAA, and fibrinogen in all participants, whereas a strong correlation of GSDMD-∆ with serum CRP and SAA was observed in the FMF group. The GSDMD-∆ did not correlate with serum IL-1β and IL-18, whereas serum IL-1β and IL-18 strongly correlated with each other. Furthermore, GSDMD-∆ did not correlate with the duration of fever in the G1-V1 and G2 groups (p>0.05).
| Normal ranges for serum acute phase reactants and interleukins: CRP <5 mg/L; SAA <0.5 mg/dL; fibrinogen <4.2 g/L; ESR <15 mm/h; IL-1β<12 pg/mL; IL-18<120 pg/mL; *Spearman’s Rho correlation (r for weak correlation: 0.2-0.4, moderate correlation: 0.4-0.6, strong correlation: ≥0.6; ^p<0.05; ^^p<0.01); GSDMD: gasdermin-D; FMF: familial Mediterranean fever; GSDMD-∆: gasdermin-D gene expression (**mean 2^(-delta delta Ct)); WBC: white blood cell count; CRP: C-reactive protein; SAA: serum amyloid-A; ESR: erythrocyte sedimentation rate. | ||||||
| Table III. Correlations between serum acute phase reactants, serum IL-1β and IL-18, and GSDMD gene expression*. | ||||||
|
|
|
|
||||
|
|
|
|
|
|
|
|
| WBC, *109/L |
|
|
|
|
|
|
| CRP, mg/L |
|
|
|
|
|
|
| SAA, mg/dL |
|
|
|
|
|
|
| Fibrinogen, g/L |
|
|
|
|
|
|
| ESR, mm/h |
|
|
|
|
|
|
| IL-1β, pg/mL |
|
|
|
|
|
|
| IL-18, pg/mL |
|
|
|
|
|
|
Discussion
To our knowledge, this is the first human study looking into changes in GSDMD-∆ during disease attacks and attack-free intervals in FMF. Our findings revealed a significant upregulation in GSDMD-∆ during FMF attacks. More than 10-fold increase in median GSDMD-∆ was observed during FMF disease attacks compared to attack- and symptom-free periods. Asymptomatic patients exhibited very low GSDMD-∆ levels, akin to healthy children. On the other hand, significantly higher GSDMD-∆ was detected in FMF attacks compared to otherwise healthy children with acute infections.
Gasdermin-D is a crucial molecule in inflammation by its direct and indirect effects on pyroptosis, significantly contributing to both acute and chronic inflammatory processes. Gasdermin-D deletion in vivo prevented spontaneous autoinflammatory attacks and systemic effects of chronic inflammation such as diminished growth, anemia, and tissue damage in the FMF knock-in mouse model.9 In this study, GSDMD-∆ significantly correlated with serum APRs in FMF patients admitted with attack symptoms. However, serum inflammatory cytokines, IL-1β and IL-18, did not differ according to the presence of FMF attack and were measured within normal ranges at both visits of the FMF group. These findings show the distinct role of GSDMD for FMF attack periods compared to serum inflammatory cytokines IL-1β and IL-18. Furthermore, GSDMD-∆ was the only laboratory test in the study that could differentiate FMF attacks from otherwise healthy children with febrile acute infections. Thus, we suggest that GSDMD be used as a biomarker of disease attacks. Although testing GSDMD-∆ can be impractical and expensive in clinical practice, further research at its protein level and cleavage can help to identify an affordable and feasible biomarker. The results of this study underline the importance of GSDMD in FMF pathogenesis.
The crucial role of GSDMD-mediated pyroptosis in the clearance of bacterial infections, particularly in sepsis, has been well-established.22,23 Gasdermin-D promotes cellular death of the infected cells, increases mucosal inflammation, and captures bacteria in pore-induced intracellular and extracellular traps.24,25 Additionally, GSDMD has been shown to restrict pathogen load in tissues and organs, control inflammation kinetics, and prevent epithelial disruption during acute Salmonella gut infection. Other gasdermins appeared dispensable for these protective functions.26 The importance of GSDMD in viral infections remains elusive although the pore-forming activity of GSDMD has been thought to be a potential therapeutic target in viral infections.27 In the present study, previously healthy children with acute infections demonstrated about a two fold increase in GSDMD-∆ compared to healthy children which was about one-fourth of the levels detected in FMF disease attacks. This finding also underpins the critical role of GSDMD in FMF.
FMF is classically accepted as an autosomal recessively inherited disease. There are few reports suggesting autosomal dominant pattern of inheritence in some patients.28 The expression of similar disease phenotypes in heterozygotes proposes a different inheritance pattern such as pseudo-dominant inheritance. Population-based clinical studies indicate no clear genotype-phenotype correlation in FMF.15,29,30 The most common MEFV gene mutations in the Turkish population are ‘exon 10’ mutations; M694V, M680I, and V726A.14 ‘Exon 10’ mutations in the MEFV gene encode the B30.2 domain of pyrin protein and lead to severe disease phenotype.31 The M694V mutation, the most common mutation found in Turkish population, is related to a severe disease phenotype and secondary amyloidosis; however, its biallelic or monoallelic presence has not been clearly linked to the severity of the disease phenotype.32,33 An ‘exon 2’ mutation, E148Q, is accepted as a polymorphism in some populations, nevertheless, it has been shown as a disease-causing mutation in the Turkish population.34,35 A recent study indicated that E148Q could induce inflammasome activation increasing the disease risk and severity rather than acting as a disease-causing monogenic mutation.36 Because of the dilemma on E148Q mutation, the only patient carrying monogenic E148Q mutation was excluded from group comparisons. In the present study, patients with biallelic and monoallelic ‘exon 10’ mutations had similar ages of disease onset, disease severity, clinical features, serum APRs, GSDMD-∆, and serum IL-1β and IL-18 levels in disease attacks and in-between attacks.
Major limitations of the study are the limited number of participants and the lack of homogeneity within the groups. Different types of infections were present in the febrile control group. Although the genetic heterogeneity among FMF patients existed, there was no difference between patients carrying biallelic and monoallelic ‘exon 10’ mutations in the MEFV gene regarding clinical, laboratory findings, and ISSF score. Furthermore, the timing for blood draw was heterogeneous for the FMF group-attack visit and the febrile control group, although it was between 8-48 hours of fever. A major strength of this study is the inclusion of pediatric FMF patients with highly penetrant MEFV mutations. The evaluation of the patients both during attack and asymptomatic visits was another important strength. Comparison with febrile and healthy controls leads to the characterization of the distinct role of GSDMD-∆ for FMF attack periods compared to APRs and serum inflammatory cytokines, IL-1β and IL-18.
Conclusion
In conclusion, this study showed the distinct role of GSDMD in FMF attacks for the first time in humans. The significant GSDMD-∆ increase during FMF attacks relative to febrile but otherwise healthy patients highlights the functional significance of GSDMD-∆ in FMF pathogenesis. We propose that it has a potential utility as a biomarker of FMF attacks. This study also supports findings that phenotypic variations are not directly related to the genotype of FMF at a molecular level, also in terms of GSDMD-∆. Further studies in larger and more homogeneous FMF cohorts and inclusion of different control groups such as patients with acute appendicitis are needed to validate these results on GSDMD that are also needed at the protein level and its cleavage.
Ethical approval
The study was approved by Clinical Research Ethics Committee of Hacettepe University Faculty of Medicine (date: 05.10.2021, number: GO21/1062).
Source of funding
The authors declare that the study is supported by the Scientific Research Projects (BAP) Coordination Unit of Hacettepe University (2021/16-27, 2022/06-66).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Sangiorgi E, Rigante D. The clinical chameleon of autoinflammatory diseases in children. Cells 2022; 11: 2231. https://doi.org/10.3390/cells11142231
- Savic S, Caseley EA, McDermott MF. Moving towards a systems-based classification of innate immune-mediated diseases. Nat Rev Rheumatol 2020; 16: 222-237. https://doi.org/10.1038/s41584-020-0377-5
- Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015; 526: 660-665. https://doi.org/10.1038/nature15514
- Sollberger G, Choidas A, Burn GL, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 2018; 3: eaar6689. https://doi.org/10.1126/sciimmunol.aar6689
- Place DE, Kanneganti TD. Cell death-mediated cytokine release and its therapeutic implications. J Exp Med 2019; 216: 1474-1486. https://doi.org/10.1084/jem.20181892
- Ben-Chetrit E. Old paradigms and new concepts in familial Mediterranean fever (FMF): an update 2023. Rheumatology (Oxford) 2024; 63: 309-318. https://doi.org/10.1093/rheumatology/kead439
- Jamilloux Y, Lefeuvre L, Magnotti F, et al. Familial Mediterranean fever mutations are hypermorphic mutations that specifically decrease the activation threshold of the Pyrin inflammasome. Rheumatology (Oxford) 2018; 57: 100-111. https://doi.org/10.1093/rheumatology/kex373
- Jorch SK, McNally A, Berger P, et al. Complex regulation of alarmins S100A8/A9 and secretion via gasdermin D pores exacerbates autoinflammation in familial Mediterranean fever. J Allergy Clin Immunol 2023; 152: 230-243. https://doi.org/10.1016/j.jaci.2023.01.037
- Kanneganti A, Malireddi RKS, Saavedra PHV, et al. GSDMD is critical for autoinflammatory pathology in a mouse model of familial Mediterranean fever. J Exp Med 2018; 215: 1519-1529. https://doi.org/10.1084/jem.20172060
- Ben-Chetrit E, Levy M. Familial Mediterranean fever. Lancet 1998; 351: 659-664. https://doi.org/10.1016/S0140-6736(97)09408-7
- Avar Aydın PÖ, Özçakar ZB, Aydın F, Karakaş HD, Çakar N, Yalçınkaya F. The characteristics of pediatric patients with familial Mediterranean fever treated with anti-interleukin-1 treatment. Turk Arch Pediatr 2022; 57: 448-452. https://doi.org/10.5152/TurkArchPediatr.2022.22039
- Avar-Aydin PO, Ozcakar ZB, Cakar N, Fitoz S, Karakas HD, Yalcinkaya F. Nutcracker syndrome: a potentially underdiagnosed cause of proteinuria in children with familial Mediterranean fever. Pediatr Nephrol 2022; 37: 1615-1621. https://doi.org/10.1007/s00467-021-05337-9
- Avar-Aydın PO, Ozcakar ZB, Aydın F, Karakas HD, Cakar N, Yalcınkaya F. The expanded spectrum of arthritis in children with familial Mediterranean fever. Clin Rheumatol 2022; 41: 1535-1541. https://doi.org/10.1007/s10067-022-06082-6
- Öztürk K, Coşkuner T, Baglan E, et al. Real-life data from the largest pediatric Familial Mediterranean Fever Cohort. Front Pediatr 2022; 9: 805919. https://doi.org/10.3389/fped.2021.805919
- Gangemi S, Manti S, Procopio V, et al. Lack of clear and univocal genotype-phenotype correlation in familial Mediterranean fever patients: a systematic review. Clin Genet 2018; 94: 81-94. https://doi.org/10.1111/cge.13223
- Yalçinkaya F, Ozen S, Ozçakar ZB, et al. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology (Oxford) 2009; 48: 395-398. https://doi.org/10.1093/rheumatology/ken509
- Shinar Y, Ceccherini I, Rowczenio D, et al. ISSAID/EMQN best practice guidelines for the genetic diagnosis of monogenic autoinflammatory diseases in the next-generation sequencing era. Clin Chem 2020; 66: 525-536. https://doi.org/10.1093/clinchem/hvaa024
- Van Gijn ME, Ceccherini I, Shinar Y, et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for systemic autoinflammatory diseases (INSAID). J Med Genet 2018; 55: 530-537. https://doi.org/10.1136/jmedgenet-2017-105216
- Neyzi O, Günöz H, Furman A, et al. Weight, height, head circumference and body mass index references for Turkish children. Çocuk Sağlığı ve Hastalıkları Dergisi 2008; 51: 1-14.
- Demirkaya E, Acikel C, Hashkes P, et al. Development and initial validation of international severity scoring system for familial Mediterranean fever (ISSF). Ann Rheum Dis 2016; 75: 1051-1056. https://doi.org/10.1136/annrheumdis-2015-208671
- Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012; 24: 69-71.
- Booty LM, Bryant CE. Gasdermin D and Beyond - Gasdermin-mediated pyroptosis in bacterial infections. J Mol Biol 2022; 434: 167409. https://doi.org/10.1016/j.jmb.2021.167409
- Salami A, Bettadapura S, Wang S. Gasdermin D kills bacteria. Microbiol Res 2023; 272: 127383. https://doi.org/10.1016/j.micres.2023.127383
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532-1535. https://doi.org/10.1126/science.1092385
- Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med 2016; 213: 2113-2128. https://doi.org/10.1084/jem.20151613
- Fattinger SA, Maurer L, Geiser P, et al. Gasdermin D is the only Gasdermin that provides protection against acute Salmonella gut infection in mice. Proc Natl Acad Sci U S A 2023; 120: e2315503120. https://doi.org/10.1073/pnas.2315503120
- Liu X, Ding S, Liu P. The roles of Gasdermin D in coronavirus infection and evasion. Front Microbiol 2021; 12: 784009. https://doi.org/10.3389/fmicb.2021.784009
- Rowczenio DM, Iancu DS, Trojer H, et al. Autosomal dominant familial Mediterranean fever in Northern European Caucasians associated with deletion of p.M694 residue-a case series and genetic exploration. Rheumatology (Oxford) 2017; 56: 209-213. https://doi.org/10.1093/rheumatology/kew058
- Yalçinkaya F, Cakar N, Misirlioğlu M, et al. Genotype-phenotype correlation in a large group of Turkish patients with familial Mediterranean fever: evidence for mutation-independent amyloidosis. Rheumatology (Oxford) 2000; 39: 67-72. https://doi.org/10.1093/rheumatology/39.1.67
- Ozturk K, Cakan M. The analysis of genotype-phenotype correlation in familial Mediterranean fever. Pediatr Int 2022; 64: e15017. https://doi.org/10.1111/ped.15017
- Chae JJ, Wood G, Masters SL, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci U S A 2006; 103: 9982-9987. https://doi.org/10.1073/pnas.0602081103
- Touitou I, Sarkisian T, Medlej-Hashim M, et al. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum 2007; 56: 1706-1712. https://doi.org/10.1002/art.22507
- Turkish FMF Study Group. Familial Mediterranean Fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore) 2005; 84: 1-11. https://doi.org/10.1097/01.md.0000152370.84628.0c
- Aydın F, Çakar N, Özçakar ZB, et al. Clinical features and disease severity of Turkish FMF children carrying E148Q mutation. J Clin Lab Anal 2019; 33: e22852. https://doi.org/10.1002/jcla.22852
- Tanatar A, Karadağ ŞG, Sönmez HE, Çakan M, Ayaz NA. Comparison of pediatric familial Mediterranean fever patients carrying only e148q variant with the ones carrying homozygous pathogenic mutations. J Clin Rheumatol 2021; 27: 182-186. https://doi.org/10.1097/RHU.0000000000001261
- Reygaerts T, Laohamonthonkul P, Hrovat-Schaale K, et al. Pyrin variant E148Q potentiates inflammasome activation and the effect of pathogenic mutations in cis. Rheumatology (Oxford) 2024; 63: 882-890. https://doi.org/10.1093/rheumatology/kead376
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.