Graphical Abstract
Abstract
Background. Food-induced anaphylaxis (FIA) is a severe form of food allergy, and literature data about multi-triggered FIA is scarce. This study aimed to evaluate the differences between multi-triggered and single-triggered food anaphylaxis in children.
Methods. The study included pediatric patients (age <18 years) who were diagnosed with FIA between January 1, 2015 and February 28, 2023. Demographic data, clinical features, laboratory findings, and allergological work-up results were evaluated from the patients’ records.
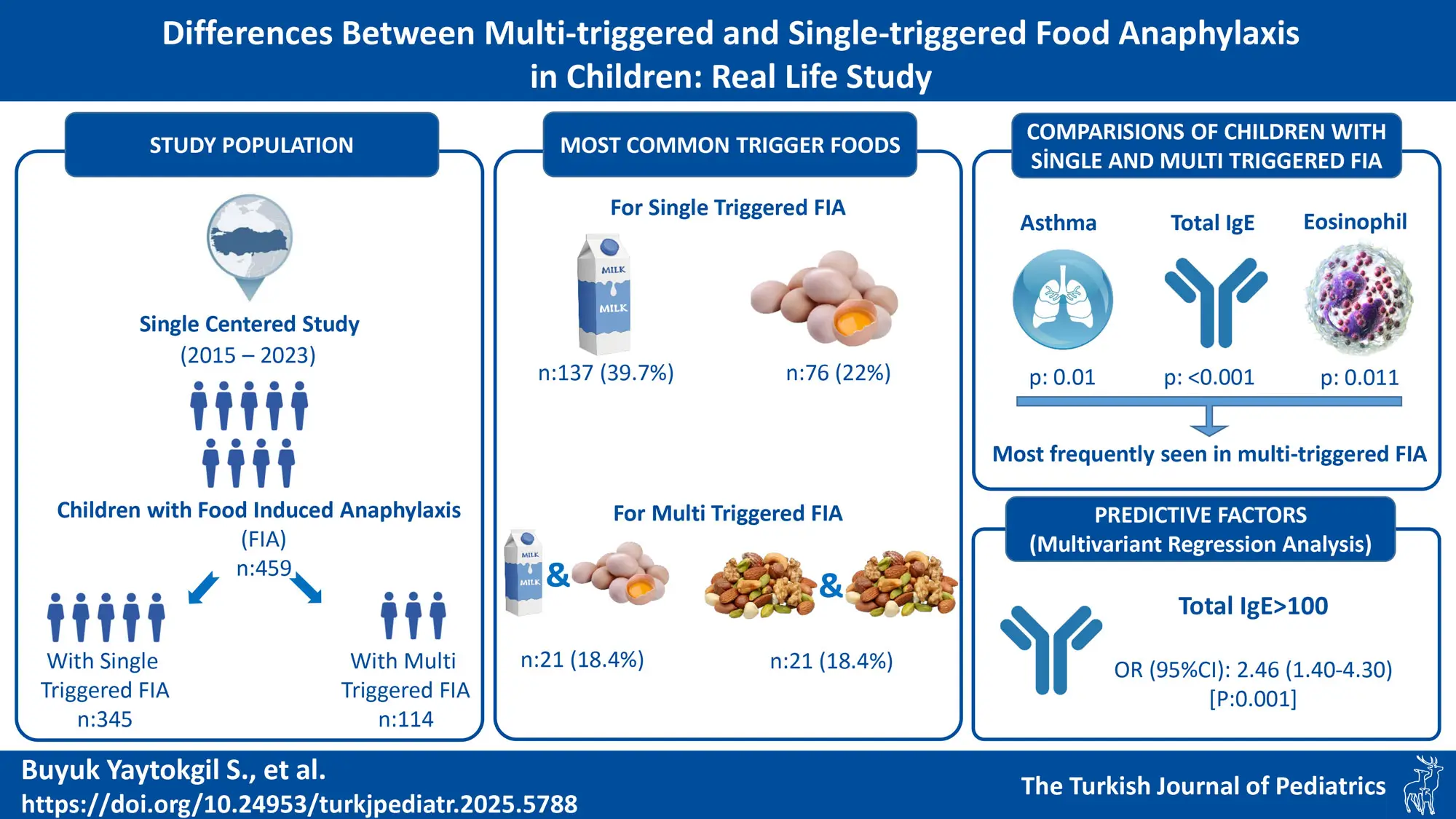
Results. A total of 459 patients (64.1% male) were evaluated. The median age at onset (first anaphylactic reaction) was 12 months (IQR:6-24 months). Food anaphylaxis with multiple foods was reported in 114 of the 459 children (24.8%). Multi-triggered FIA was most commonly associated with combinations of milk and egg (n=21) and tree nut and tree nut (n=21). Atopic disease, asthma, higher total IgE level, and higher eosinophil count were more frequent in patients with multi-triggered FIA. In multivariate regression analysis, total IgE >100 IU/mL was identified as a predictive factor for multi-triggered FIA (Odds ratio (95% CI): 2.46 (1.40-4.30), p=0.001).
Conclusions. FIA with multiple trigger foods was detected in approximately a quarter of the children with FIA. Multi-triggered FIA was associated with higher rates of atopic disease, asthma, eosinophilia, and increased total IgE levels. A total IgE level higher than 100 IU/mL was a risk factor for multi-triggered FIA. This suggests that high IgE levels may be a warning sign for clinicians to be vigilant for multiple food triggers in the screening and follow-up of FIA patients.
Keywords: anaphylaxis, food induced anaphylaxis, multiple triggered, single triggered, total IgE
Introduction
Anaphylaxis is a severe, life-threating disease and foods are the main triggers of anaphylaxis in children.1-5 Food-induced anaphylaxis (FIA) can sometimes occur with more than one food, which is referred to as multi-triggered FIA. However, there is limited information about this in the literature, and the true prevalence of multi-triggered FIA remains unclear. Identifying genuine cases of anaphylaxis to multiple foods is challenging due to the severity of the reaction, fear of oral challenge testing, and/or cross-reactivity. McIntyre et al. reported that approximately a third of anaphylaxis cases occurred with multiple substances, and approximately two-thirds (66%) of the reactions in patients with multiple allergies were related to multiple foods.6
A recent study indicated that multi-food allergic individuals were epigenetically distinct from single-food allergic individuals.7 Other studies reported that patients with multiple food allergy (MFA) had more food allergy-related adverse events8, as well as more severe food-induced allergic reactions and more frequent emergency department visits.9 Patients with MFA were also reported to have an increased prevalence of atopic comorbidities and asthma morbidity10-12, while highly atopic children may be prone to having allergies to multiple foods.13 MFA can result in increased accidental ingestions and may cause fatal/near-fatal reactions.9 Several studies reported that MFA imposes a greater physical health impact, quality of life impairment, and economic burden than single food allergy (SFA).11,13,14 These impacts may be exacerbated in patients with multi-triggered FIA because of the rigid elimination requirements and later development of tolerance. Although data about multi-triggered FIA are scarce, we hypothesized that these patients may exhibit differences from those with single-triggered FIA, like in MFA.
It is known that having one atopic disease can lead to having another; thus, a SFA may predispose to additional food allergies.15 However, we are aware of no study published to date that demonstrates predictive factors for multi-triggered FIA.
In the present study, we examined the differences in the characteristics of children with FIA to single and multiple foods and assessed the value of certain factors in predicting multi-triggered FIA.
Materials and Methods
In this single-center retrospective study, children (age <18 years) who presented to our pediatric allergy and immunology clinic with FIA between 2015 and 2023 were identified by screening patient records in the hospital’s electronic database. If the records were incomplete, we contacted the patient’s family to obtain missing data.
We recorded patient data in a standardized form that included their demographic and clinical characteristics, laboratory results (eosinophil count and percentage; tryptase levels and total IgE levels at presentation), and allergological work-up results (baseline specific IgE [sIgE] levels, skin prick test (SPT) wheal diameter, and oral food challenge (OFC) results. Results closest to the anaphylaxis reaction were recorded.
FIA was defined according to the criteria established by the European Academy of Allergy and Clinical Immunology (EAACI) task force position papers on childhood anaphylaxis management.1,16 All cases were evaluated by pediatric allergology physicians based on clinical history and complementary allergy tests (skin prick test / sIgE / Tryptase levels / open food challenge) cases were deemed to involve anaphylaxis triggered by a specific food when these assessments confirmed the diagnosis. Tryptase levels were considered if the patient was admitted within six hours after the reaction or if the reaction occurred during an open food challenge. Open food challenge tests were conducted in appropriate cases, provided parental informed consent was obtained and the case was considered non severe.
Trigger foods were identified through reaction history and allergological testing, including SPT, serum sIgE levels (measured using the Immulite® 2000 System; Siemens Healthcare Diagnostics, Tarrytown, New York), and/or OFC tests. sIgE levels were regarded as positive when equal to or greater than 0.35 kU/L. SPT was performed at least four to six weeks after the anaphylactic reaction, with antihistamines discontinued one week before testing. SPT was conducted using commercial preparations (ALK, Abello, Madrid, Spain) or fresh foods (e.g., vegetables, fruit, and fish) via the prick-to-prick method following EAACI guidelines.17 SPT was performed on the back in younger children and on the inner forearm in older children. Maximum horizontal and vertical wheal diameters were measured after 15 minutes, with a reaction considered positive if the mean diameter exceeded the negative control by at least 3 mm.
After obtaining informed parental consent; open OFCs were performed in our allergy unit under the supervision of an allergist according to EAACI guidelines.17 A stepwise OFC was preferred for milk (baked, then yogurt, then milk) and eggs (baked, then boiled). In the open OFC, the culprit food was freshly prepared and administered orally in incrementally increasing doses every 15 minutes. OFCs were considered positive if objective symptoms occurred within two hours of the last challenge dose.
Trigger foods were categorized into eight groups: milk, egg, treenuts, legumes, seafood, fruits/vegetables, seeds, and other. Treenuts included hazelnuts, walnuts, cashews, almonds, and pistachios. Legumes comprised lentils, peanuts, chickpeas, beans, peas, and soy. The seeds group included sesame, poppy, sunflower, and pumpkin seeds. Fruits/vegetables included banana, kiwi, potato, peach, coconut, strawberry, tomato, apple, melon, olive, and red pepper. The “other” category encompassed honey, meats (chicken and beef), sumac, cinnamon, and wheat.
Anaphylaxis triggered by more than one food was classified as multi-triggered FIA.18 However, due to the retrospective nature of the study, cross-reactivity could not be ruled out and food-specific components could not be measured in our hospital. Nevertheless, as all included patients met EAACI anaphylaxis criteria, demonstrated clinical reactivity and had confirmed allergens based on sIgE, SPT and/or OFC, all cases of anaphylaxis involving multiple foods were included in the statistical analysis as part of the “multi-triggered FIA” group. This classification applied even when multiple tree nuts or legumes were involved.18 Patients with multiple food allergies who experienced anaphylaxis triggered by only one food were classified as having single triggered anaphylaxis.
All patients were assessed for other atopic diseases according to international guidelines by a pediatric allergy immunology specialist.19-21 Patients were also evaluated for mastocytosis based on clinical history (recurrent, venom-induced or unexplained anaphylaxis), physical examination (typical skin lesions, hepatosplenomegaly) and/or baseline tryptase levels.
Ethics
This study protocol was reviewed and approved by Ankara Bilkent City Hospital No. 2 Clinical Research Ethics Committee (date: 15.03.2023, number: E2-23-3593).
Statistics
SPSS version 22.0 (IBM Corp, Armonk, NY) was used for statistical analyses. The data were presented as median and interquartile ranges (IQR) because the data were not normally distributed. For comparisons of groups, we used the chi-squared (χ2) and Fisher’s exact tests for qualitative variables and the Mann-Whitney U and Wilcoxon rank sum tests for quantitative variables. Univariate and multivariate analysis were used to identify risk factors for multi-triggered FIA. Results with P values < 0.005 were considered statistically significant.
Results
A total of 459 patients (599 reactions) met the inclusion criteria. The median age at the time of the first anaphylactic reaction was 12 months (IQR: 6-24 months) and 64.21% (n = 294) were male. The baseline characteristics of the patients are summarized in Table I and characteristics of the reactions are delineated in Table II.
| IQR, interquartile range; NA, not analyzed. | ||||
| Table I. Demographic characteristics of children and comparison between anaphylaxis cases triggered by single versus multiple food allergens. | ||||
| Parameters |
|
(n: 345) |
(n: 114) |
|
| Age at first anaphylaxis (months), median (IQR) |
|
|
|
|
| Male gender, n (%) |
|
|
|
|
| Atopic diseases, n (%) |
|
|
|
|
| Asthma |
|
|
|
|
| Atopic dermatitis |
|
|
|
|
| Allergic rhinitis |
|
|
|
|
| History of family atopic disease, n (%) |
|
|
|
|
| Multiple food allergy |
|
|
|
|
| Co-anapyhylaxis with different foods, n (%) |
|
|
|
|
| Recurrent anaphylaxis with same food, n (%) |
|
|
|
|
| Anaphylaxis was the first symptom, n (%) |
|
|
|
|
| Total IgE (IU/mL), median (IQR) |
|
|
|
|
| Eosinophil count (×109/L), median (IQR) |
|
|
|
|
| GIS, gastrointestinal system; IQR: interquartile range, sIgE: specific immunoglobulin E, SPT: skin prick test. | |||
| Table II. Characteristics of reactions (n: 599). | |||
|
|
|
|
|
| Types of food, n (%) | |||
| Milk |
|
|
|
| Egg |
|
|
|
| Tree nut |
|
|
|
| Hazelnut |
|
|
|
| Walnut |
|
|
|
| Pistachio |
|
|
|
| Cashew |
|
|
|
| Almond |
|
|
|
| Legume |
|
|
|
| Lentil |
|
|
|
| Peanut |
|
|
|
| Chickpea |
|
|
|
| Beans |
|
|
|
| Peas |
|
|
|
| Soy |
|
|
|
| Fish |
|
|
|
| Fruits/vegetables |
|
|
|
| Banana |
|
|
|
| Kiwi |
|
|
|
| Potatoes |
|
|
|
| Peach |
|
|
|
| Coconut |
|
|
|
| Strawberry |
|
|
|
| Tomato |
|
|
|
| Apple |
|
|
|
| Melon |
|
|
|
| Red pepper |
|
|
|
| Olive |
|
|
|
| Seeds |
|
|
|
| Sesame |
|
|
|
| Poppy |
|
|
|
| Sun flower seed |
|
|
|
| Pumpkin |
|
|
|
| Other |
|
|
|
| Meats (5 chicken, 5 beef) |
|
|
|
| Honey |
|
|
|
| Wheat |
|
|
|
| Sumac |
|
|
|
| Cinnamon |
|
|
|
| Symptoms, n (%) | |||
| Skin |
|
|
|
| GIS |
|
|
|
| Respiratory |
|
|
|
| Neurological |
|
|
|
| Cardiovascular |
|
|
|
| Severity of anaphylaxis, n (%) | |||
| Mild |
|
|
|
| Moderate |
|
|
|
| Severe |
|
|
|
| Reaction time (minute), median (IQR) |
|
|
|
Multi-triggered FIA
Food anaphylaxis involving more than one food was observed in 114 of the 459 children (24.8%), accounting for 254 reactions. Among these, ninety-two patients experienced anaphylactic reactions at different times with two different foods, 19 with three different foods, 2 patients with four different foods, and 1 patient with five different foods (Supplementary Table: Triggered foods in patients with multi-triggered FIA). The most common food combinations associated with multi-triggered FIA were milk/egg (n=21) and treenut/treenut (two different treenut co-anaphylaxis) (n=21; with walnut/hazelnut (n=15) being the most frequent pairing; combinations are shown in the Supplementary Table).
Children with multi-triggered FIA had higher rates of atopic disease and asthma, as well as elevated IgE and eosinophil levels compared to those with single-triggered FIA, (p= 0.049, p= 0.010, p= <0.001, p=0.010, respectively). The similarities and differences between these groups are detailed in Table I.
Multivariate regression analysis identified, a total IgE level >100 IU/mL as a risk factor for multi-triggered FIA (Odds ratio [95% CI]: 2.46 [1.40-4.30], p=0.001, Table III).
| CI, confidence interval; IgE, immunglobulin E; OR, odds ratio. | ||||||
| Table III. Predictive factors for food-induced anaphylaxis with multiple triggering foods. | ||||||
| Parameters |
|
|
||||
|
|
|
|
|
|
|
|
| Gender (male) |
|
|
|
|||
| >2 years of age |
|
|
|
|
|
|
| Presence of other atopic disease |
|
|
|
|
|
|
| Atopic dermatitis |
|
|
|
|||
| Asthma |
|
|
|
|
|
|
| Allergic rhinitis |
|
|
|
|
|
|
| Family atopic disease history |
|
|
|
|||
| Total IgE >100 IU/mL |
|
|
|
|
|
|
| Eosinophil ≥400×109/L |
|
|
|
|
|
|
Severity of reactions
Among the 345 patients with single triggered FIA; and 57.1% (n=197) of reactions were moderate; 26.1% (n=90) of them were mild and 16.8 % (n= 58) were severe. In contrast, the 114 patients with multi-triggered FIA experienced 254 reactions; of which 67.7% (n=172) were moderate, 20.9 % (n:53) were mild and 11.4% (n=29) were severe.
Unusual triggers
Four patients experienced anaphylaxis after consuming honey, none of whom had pollen atopy, although one had a history of venom anaphylaxis. Five patients had anaphylaxis triggered by potatoes with all reactions occurring after consuming baked potatoes. Two patients experienced anaphylaxis triggered by red meat, both reacting to well-cooked meat despite no known exposure to ticks.
Discussion
This single-centered, real-life study, examines the differences between multi-triggered and single-triggered food anaphylaxis in children. Multi-triggered FIA was detected in 24.8% of the patients with FIA. Patients with multi-triggered FIA were more likely to have atopic disease, asthma, higher total IgE levels, and higher eosinophil counts. Additionally, a total IgE level above 100 IU/mL was associated with an increased risk of multi-triggered FIA.
Food allergens are common triggers of anaphylaxis in children.1,2,4,5 While the exact prevalence of FIA remains uncertain, non-fatal FIA reactions have been reported at an incidence of 0.5-16 per 100000 person-years.22 In a study on epinephrine administration for life-threatening allergic reactions in school settings, McIntyre et al. reported that over one-third (36%) of reactions occurred in individuals allergic to multiple substances, with approximately two-thirds (66%) of these reactions involving multiple foods.6 Although there is limited data about the prevalence of multi-triggered FIA, several studies reported MFA in 30-40% of children with food allergy.9,18,23 In the current study, 24.8% of FIA cases involved multiple trigger foods.
Individual food allergen types in FIA may vary according to culture and population.22 The most commonly reported triggers in FIA included cow’s milk, hen’s egg, peanut, tree nuts, shellfish, and fish.2 McIntyre et al. reported that among patients who reported multiple allergies, the most common allergens listed were tree nuts (54%) and peanuts (51%).6 In our study, multi-triggered FIA was most frequently associated with milk and eggs or a combination of tree nuts.
Many studies have investigated co-allergy and co-sensitization to treenuts. The most common treenut co-allergens vary by country depending on consumption patterns, with pistachio/cashew and walnut/pecan among the most frequently reported combinations.24,25 In contrast, we observed in this study that walnut and hazelnut were the most common tree nut combinations associated with multi-triggered FIA. This is not surprising, considering that walnut and hazelnut are the most consumed treenuts in our country.26 Another study from our country showed that walnut-allergic children have a higher rate of concomitant hazelnut allergy.25 Furthermore, unlike the aforementioned studies, our study focused on the most severe cases (anaphylaxis), and reaction severity may differ according to the combination of foods. Two components of hazelnut, Cor a 9 and Cor a14, are members of a protein family known to cause severe anaphylactic reactions.24
In our study, milk/egg was the most common combination of anaphylaxis-inducing foods overall. This may be attributed to the age distribution of our sample, which was predominantly composed of infants. The intake ratio of a food is a determining factor in the development of allergic reactions24, and infants typically consume milk and egg more than other types of foods. We also observed that egg in particular was the most common co-allergen food in children with multi-triggered FIA. A mouse model study illustrated that primary ovalbumin sensitization enhanced sensitization to a secondary unrelated allergen (latex).27 Our findings may support this observation. In addition, Cetinkaya et al. reported egg white as the most common co-allergen other than tree nut/peanut in patients with tree nut/peanut allergy, but only 41.4% of the patients in their study had anaphylactic reactions.25 Masthoff et al. reported that peanut allergy was common among hazelnut-sensitized patients but was not primarily due to IgE cross-reactivity. They indicated that it may be a result of having an atopic predisposition, and being exposed to tree nuts and peanuts most likely increased the risk for co-sensitization to both foods.28 They also proposed that T-cell cross-reactivity could contribute to this phenomenon.28 Further research is needed to understand why egg or tree nuts tend to cause multi-triggered FIA as observed in our study and previous investigations.
A study indicated that individuals with MFA exhibited epigenetic differences from individuals allergic to a single food, detected as variations in DNA methylation between these groups.7 Therefore, there may also be some epigenetic differences between multi-triggered FIA and single-triggered FIA. Furthermore, certain immunological differences were reported previously between single- and multi-triggered food allergies.8,27,29 It is well established that DOCK8 deficiency is likely associated with anaphylaxis with multiple foods.29 However, there is limited information about the pathophysiologic, genetic/epigenetic, and clinical differences between single-triggered and multi-triggered FIA. Understanding these differences and identifying predisposing factors for multi-triggered FIA could aid in prevention and management. Previous studies have shown that MFA is linked to greater impairment in quality of life, higher rates of concomitant allergic disease, and increased reaction severity.9,11,18 Warren et al. reported that the prevalence of physician-diagnosed atopic comorbidities increased significantly as the number of reported convincing food allergies increased.18 Wang also stated that highly atopic children may be at greater risk of developing allergies to multiple foods13, while Hill et al. reported that individuals with MFA were at increased risk of developing asthma and rhinitis compared to patients with a SFA.11 In our study, concomitant atopic disease, especially asthma was more frequent in children with multi-triggered FIA. Asthma may contribute to the development of anaphylaxis by increasing the severity of reactions. Moreover, we detected that total IgE levels and eosinophil counts were higher in children with multi-triggered FIA than in those with single-triggered FIA. Similarly, Blumchen et al. reported that total IgE levels and Th2 responses were higher in double allergen sensitized mice than single allergen sensitized mice, and that the strength of the primary sensitization is an important factor in increasing Th2 responses to the secondary allergen.27
Data about predictors of multi-triggered FIA are also scarce. In previous tree nut studies investigating multiple nut allergies, patient age was reported to be an important factor influencing the prevalence of MFA.2,24,30 It was also noted that because the number of nuts consumed increased with age, older age may be associated with multiple nut allergies.30 Çetinkaya et al. reported that a current age of 6-10 years and a family history of atopy were risk factors for multiple tree nut/peanut allergies.25 Waren et al. determined that MFA was more prevalent among non-Hispanic Black and Asian children compared to non-Hispanic White children.18 Ruran et al. suggested that social and environmental risk factors and other comorbidities may also predispose patients to develop MFA.9 As mentioned above, atopic children may have a higher risk for MFA.13 In addition, Allen et al. reported that infants with vitamin D insufficiency were more prone to developing MFA rather than SFA.29 Unlike these previous studies, we specifically investigated predictors of multi-triggered FIA, rather than MFA, and found that total IgE levels above 100 IU/mL were associated with multi-triggered FIA. This suggests that for food-allergic children with high total IgE levels, clinicians should be more vigilant for recurrent anaphylaxis with other types of foods and may follow them more closely. However, the causality of this relationship remains unclear, as elevated IgE levels may both contribute to and result from FIA, as seen in DOCK8-deficient patients. We hope that our results may guide new studies on multi-triggered FIA in terms of its prevalence, mechanisms, characteristics, and management. However, IgE levels alone are insufficient to predict multi-triggered FIA; as they are influenced by factors such as allergen exposure frequency and duration. Therefore, a large-scale cohort study is necessary to better understand these relationships.
Component testing can be useful in distinguishing between cross-reactivity and true sensitization, especially in patients with allergies to multiple foods such as nuts. However, since these tests were not performed on the patients in our study, we could not make a clear distinction between cross-reactivity and sensitization. This is the most important limitation of our study. Another limitation is that we classified patients into single- or multi-triggered groups based on the number of anaphylaxis episodes. However, confounding factors—such as protective parental behaviors (e.g., eliminating suspicious allergens from the child’s diet) or the child’s age (e.g., not yet exposed to certain foods)—may have influenced the occurrence of new anaphylaxis events, potentially limiting our findings.
Additionally, baseline serum tryptase levels were not available for all patients, which restricted our ability to assess the potential presence of systemic mastocytosis. However, systemic mastocytosis is extremely rare in children, with most pediatric cases limited to cutaneous forms that typically resolve spontaneously.31,32 None of our patients exhibited clinical features suggestive of systemic mastocytosis, such as characteristic skin lesions, hepatosplenomegaly, or unexplained recurrent anaphylaxis. In the absence of clinical signs indicating systemic involvement, we did not pursue further diagnostic evaluations, such as bone marrow biopsy, in accordance with current guidelines.32,33
The strongest aspect of this research is that it is a single-centered, real-life FIA study. Since there are a limited number of studies in the literature reporting the differences between single and multiple food anaphylaxis, we believe that our study makes a significant contribution to the literature.
Furthermore, our study highlights the considerable rate of multi-triggered FIA among anaphylaxis cases. This should encourage new studies on this subject; because further longitudinal, multi-layer, case-control and molecular-based studies are needed.
In conclusion, multi-triggered FIA was detected in approximately a quarter of children with FIA. Higher rates of atopic disease and asthma and higher levels of total IgE and eosinophilia were observed in patients with multi-triggered FIA when compared with children with single-triggered FIA. Moreover, higher total IgE was found to be a significant predictor of multi-triggered FIA.
Ethical approval
The study was approved by Ankara Bilkent City Hospital No. 2 Clinical Research Ethics Committee (date: 15.03.2023, number: E2-23-3593).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy 2022; 77: 357-377. https://doi.org/10.1111/all.15032
- Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J 2020; 13: 100472. https://doi.org/10.1016/j.waojou.2020.100472
- González-Díaz SN, Villarreal-González RV, Fuentes-Lara EI, et al. Knowledge of healthcare providers in the management of anaphylaxis. World Allergy Organ J 2021; 14: 100599. https://doi.org/10.1016/j.waojou.2021.100599
- Golden DBK, Wang J, Waserman S, et al. Anaphylaxis: a 2023 practice parameter update. Ann Allergy Asthma Immunol 2024; 132: 124-176. https://doi.org/10.1016/j.anai.2023.09.015
- Samady W, Trainor J, Smith B, Gupta R. Food-induced anaphylaxis in infants and children. Ann Allergy Asthma Immunol 2018; 121: 360-365. https://doi.org/10.1016/j.anai.2018.05.025
- McIntyre CL, Sheetz AH, Carroll CR, Young MC. Administration of epinephrine for life-threatening allergic reactions in school settings. Pediatrics 2005; 116: 1134-1140. https://doi.org/10.1542/peds.2004-1475
- Imran S, Neeland MR, Koplin J, et al. Epigenetic programming underpins B-cell dysfunction in peanut and multi-food allergy. Clin Transl Immunology 2021; 10: e1324. https://doi.org/10.1002/cti2.1324
- Neeland MR, Andorf S, Dang TD, et al. Altered immune cell profiles and impaired CD4 T-cell activation in single and multi-food allergic adolescents. Clin Exp Allergy 2021; 51: 674-684. https://doi.org/10.1111/cea.13857
- Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011; 128: e9-17. https://doi.org/10.1542/peds.2011-0204
- Ruran HB, Bartnikas LM. Multifood allergy: more than meets the eye. Ann Allergy Asthma Immunol 2023; 130: 540-541. https://doi.org/10.1016/j.anai.2023.01.038
- Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr 2016; 16: 133. https://doi.org/10.1186/s12887-016-0673-z
- Park JH, Ahn SS, Sicherer SH. Prevelance of allergy to multiple versus single foods in a pediatric food allergy referal practice. J Allergy Clin Immunol 2010; 125(Suppl. 1): AB216. https://doi.org/10.1016/j.jaci.2009.12.843
- Wang J. Management of the patient with multiple food allergies. Curr Allergy Asthma Rep 2010; 10: 271-277. https://doi.org/10.1007/s11882-010-0116-0
- Worm M, Francuzik W, Renaudin JM, et al. Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from The European Anaphylaxis Registry. Allergy 2018; 73: 1322-1330. https://doi.org/10.1111/all.13380
- Foong RX, du Toit G, Fox AT. Asthma, food allergy, and how they relate to each other. Front Pediatr 2017; 5: 89. https://doi.org/10.3389/fped.2017.00089
- Muraro A, Roberts G, Clark A, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy 2007; 62: 857-871. https://doi.org/10.1111/j.1398-9995.2007.01421.x
- Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 2014; 69: 1008-1025. https://doi.org/10.1111/all.12429
- Warren CM, Aktas ON, Manalo LJ, Bartell TR, Gupta RS. The epidemiology of multifood allergy in the United States: a population-based study. Ann Allergy Asthma Immunol 2023; 130: 637-648.e5. https://doi.org/10.1016/j.anai.2022.12.031
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980; 60(Suppl. 92): 44-47. https://doi.org/10.2340/00015555924447
- Global Initiative for Asthma. Global strategy for asthma management and prevention 2024. Available at: https://ginasthma.org/2024-report/
- Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol 2017; 140: 950-958. https://doi.org/10.1016/j.jaci.2017.03.050
- Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am 2012; 32: 165-195. https://doi.org/10.1016/j.iac.2011.10.002
- Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 2018; 142: e20181235. https://doi.org/10.1542/peds.2018-1235
- Midun E, Radulovic S, Brough H, Caubet JC. Recent advances in the management of nut allergy. World Allergy Organ J 2021; 14: 100491. https://doi.org/10.1016/j.waojou.2020.100491
- Cetinkaya PG, Buyuktiryaki B, Soyer O, Sahiner UM, Sackesen C, Sekerel BE. Phenotypical characterization of tree nuts and peanut allergies in east Mediterranean children. Allergol Immunopathol (Madr) 2020; 48: 316-322. https://doi.org/10.1016/j.aller.2019.07.005
- Tüm Kuruyemiş Sanayicileri ve İş Adamları Derneği (TÜKSİAD). 2024 TÜKSİAD dergi. Available at: https://www.tuksiad.org/Webkontrol/SayfaYonetimi/Dosyalar/2024-tuksiad-dergi_sayfa_g673_tTyLhbqy.pdf
- Blumchen K, Gerhold K, Schwede M, et al. Effects of established allergen sensitization on immune and airway responses after secondary allergen sensitization. J Allergy Clin Immunol 2006; 118: 615-621. https://doi.org/10.1016/j.jaci.2006.04.054
- Masthoff LJ, van Hoffen E, Mattsson L, et al. Peanut allergy is common among hazelnut-sensitized subjects but is not primarily the result of IgE cross-reactivity. Allergy 2015; 70: 265-274. https://doi.org/10.1111/all.12554
- Su HC, Jing H, Angelus P, Freeman AF. Insights into immunity from clinical and basic science studies of DOCK8 immunodeficiency syndrome. Immunol Rev 2019; 287: 9-19. https://doi.org/10.1111/imr.12723
- Clark AT, Ewan PW. The development and progression of allergy to multiple nuts at different ages. Pediatr Allergy Immunol 2005; 16: 507-511. https://doi.org/10.1111/j.1399-3038.2005.00310.x
- Matito A, Carter M. Cutaneous and systemic mastocytosis in children: a risk factor for anaphylaxis? Curr Allergy Asthma Rep 2015; 15: 22. https://doi.org/10.1007/s11882-015-0525-1
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis 2021; 13: e2021069. https://doi.org/10.4084/MJHID.2021.069
- Akin C. How to evaluate the patient with a suspected mast cell disorder and how/when to manage symptoms. Hematology Am Soc Hematol Educ Program 2022; 2022: 55-63. https://doi.org/10.1182/hematology.2022000366
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















