Graphical Abstract
Abstract
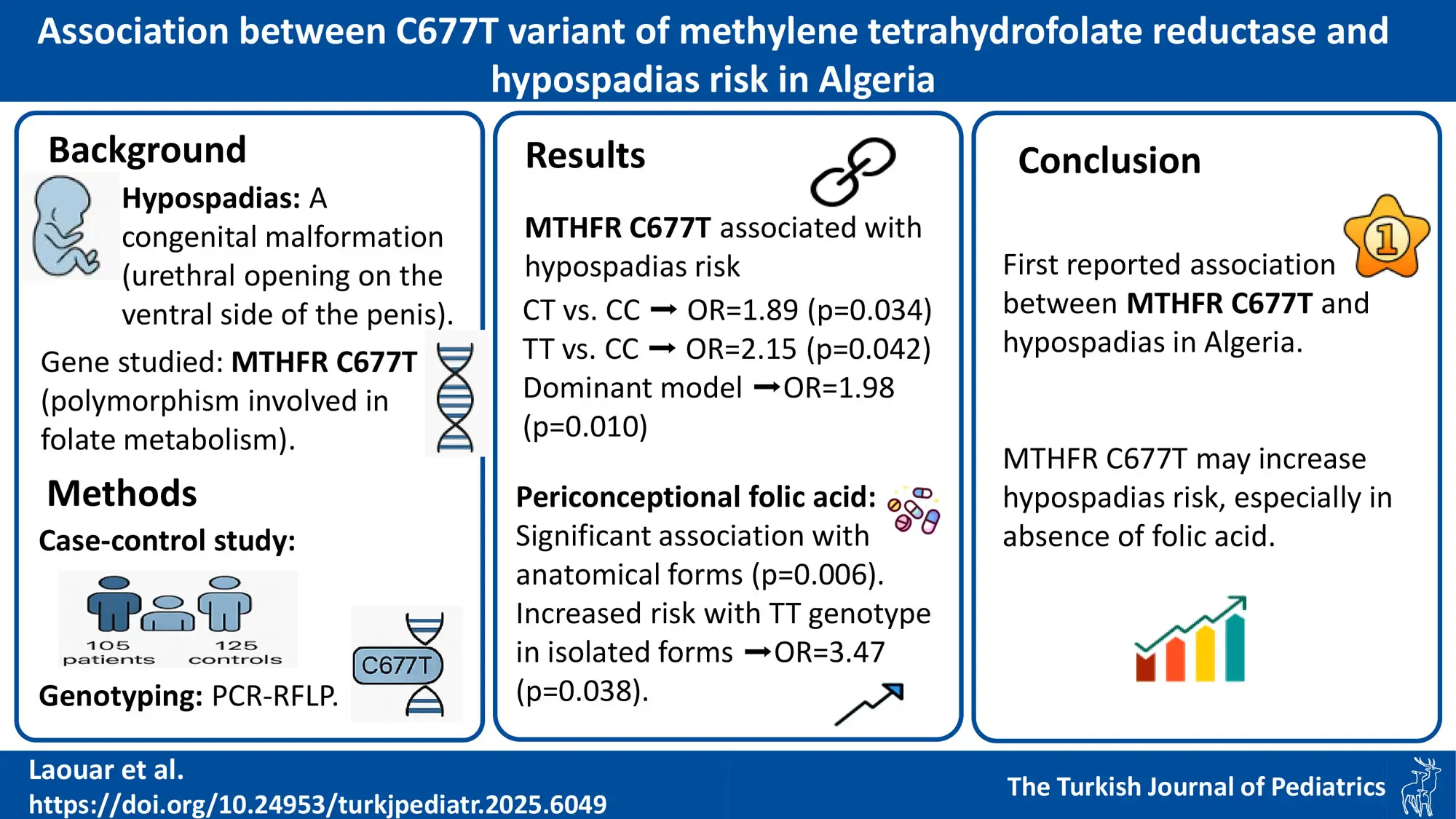
Background. Hypospadias, a congenital condition characterized by the urethral opening being on the underside of the penis, has received limited attention in its association with the MTHFR C677T variant. Given the crucial role of folate metabolism in embryonic development, and the involvement of the MTHFR C677T polymorphism in folate metabolism, this study aims to investigate whether this variant contributes to the risk of hypospadias in an Algerian population.
Methods. This case-control study included 105 patients with hypospadias and 125 controls. Genotyping of the MTHFR gene C677T variant was performed using polymerase chain reaction-restriction fragment length polymorphism.
Results. A statistically significant difference in the genotype distribution of the MTHFR C677T variant between patients with hypospadias and controls was revealed. The significance was observed in the codominant genetic model CT vs. CC (p=0.034, odds ratio [OR]: 1.89, 95% CI: 1.04-3.44) and TT vs. CC (p=0.042, OR: 2.15, 95% CI: 1.02-4.53), as well as in the dominant model CC vs. CT+TT (p=0.010, OR: 1.98, 95% CI: 1.17-3.35).
Maternal periconceptional folic acid supplement intake showed a significant association with the anatomical types of hypospadias in relation to the MTHFR C677T genotypes when folic acid was taken (p=0.006). Furthermore, a significant association was observed with the TT genotype in isolated hypospadias cases (p=0.038, OR=3.47, 95% CI: 1.03-11.68), suggesting a potential role of folic acid in modifying hypospadias risk.
Multiple logistic regression analysis identified intrauterine growth restriction, gestational hypertension, residency, and the MTHFR C677T variant as independent potential risk factors for hypospadias development (p-values: 0.030, 0.016, 0.040, and 0.045, respectively).
Conclusions. This study reports, for the first time, an association between the MTHFR gene C677T variant and hypospadias in the Algerian population. The findings suggest a strong association between the MTHFR C677T variant and susceptibility to hypospadias. Identified risk factors such as intrauterine growth restriction, gestational hypertension, rural residency, and the MTHFR C677T variant contribute valuable insights into the multifaceted etiology of hypospadias in this population.
Keywords: hypospadias, MTHFR, C677T, risk factors, folic acid, polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP)
Introduction
Hypospadias, a congenital malformation affecting the male genitourinary tract, results from partial urethral fold fusion during early fetal development.1 It is among the most prevalent congenital anomalies, affecting approximately one in 200–300 newborn boys.2 The condition’s severity stratifies it into three degrees: milder anterior forms present with the urethral opening at the glandular or subcoronal position of the penis, while more severe middle hypospadias has the meatus opening at the midshaft of the penis, and the most severe posterior forms display penoscrotal, scrotal, or perineal openings.3 Although most cases of hypospadias occur independently, associated abnormalities include unilateral or bilateral cryptorchidism and micropenis.4 While the precise causes of hypospadias remain incompletely understood, it is believed that a confluence of environmental and genetic factors contributes to its development.5
Folic acid plays an essential role in DNA (deoxyribonucleic acid) methylation processes, which are crucial for harmonious embryonic development. Adequate maternal folic acid supplementation, especially during the periconceptional period, is paramount for preventing congenital anomalies.6,7 This supplementation is associated with a lower risk of neural tube defects, as well as cardiac, urinary tract, and limb defects.8 Folic acid supplementation increases the activity of methylenetetrahydrofolate reductase (MTHFR), an enzyme involved in folate metabolism, which helps to reduce the risk of birth defects by ensuring proper folate metabolism.9
The MTHFR C677T gene variant is a functional variant in the MTHFR gene, leading to reduced enzymatic activity and elevated blood homocysteine levels.10 Research investigating the role of MTHFR C677T in DNA methylation has yielded varying results, suggesting that the MTHFR C677T variant may have diverse effects on DNA methylation depending on the cell type, folate availability, and other factors.11,12 Additionally, the MTHFR C677T variant has been extensively studied in relation to various conditions, including cardiovascular ischemic risk, neural tube defects, and cleft lip and palate.13-15 However, only one study has investigated the relationship between the MTHFR C677T variant and hypospadias, indicating a significant gap in the literature on this topic.
A case-control study involving 855 hypospadias cases and 713 controls found that hypospadias was not generally associated with folic acid use, the MTHFR C677T polymorphism, or their interaction. However, the study identified an association between middle hypospadias and the absence of folic acid supplement use, especially in infants carrying the CT/TT genotype.16 Understanding the broad impact of this variant highlights the importance of studying its potential contribution to non-syndromic hypospadias.17
Given the limited research on the relationship between the MTHFR C677T variant and hypospadias, with only one study currently available, this study aims to explore the association between the MTHFR C677T polymorphism and hypospadias, taking into account maternal periconceptional use of folic acid supplements. We hypothesize that the C677T variant is associated with an increased risk of hypospadias and that folic acid supplementation may modulate this effect. To investigate this hypothesis, our study intended to evaluate the connection between the MTHFR C677T variant and hypospadias in Algerian children.
Materials and Methods
Study population
The study comprised 230 participants, categorized into two groups: 105 patients with a diagnosis of hypospadias and 125 control subjects. Patient recruitment occurred across two locations: the endocrinology-diabetology service of the University Hospital Center in Constantine and the pediatric surgery department specialized in mother and child care at El Eulma-Setif.
For the case group, inclusion criteria were: male patients diagnosed with hypospadias, aged under 15 years, with available biological samples and complete clinical data. All patients included had a confirmed 46,XY karyotype.
Exclusion criteria were the presence of syndromic features, chromosomal abnormalities, ambiguous genitalia, or incomplete medical and/or genetic data. Ambiguous genitalia were defined as the presence of external genitalia that do not allow an assignment as male or female at birth. Although endocrine testing was not systematically performed, all patients underwent a detailed clinical examination by experienced pediatric urologists, and none showed signs of genital ambiguity or endocrine disorders.The case group included both isolated hypospadias and hypospadias associated with non-syndromic anomalies such as intrauterine growth restriction (IUGR), micropenis, cryptorchidism, and penile curvature.
For the control group, inclusion criteria were: male children without any congenital malformations, especially no history of hypospadias or other urogenital anomalies, matched in age range with the case group, and with available biological samples and clinical information. Exclusion criteria included a family history of hypospadias or congenital anomalies, known or suspected genetic disorders, or incomplete medical records.
Detailed medical records were thoroughly examined for all cases to identify familial history, evaluate the severity of hypospadias, assess clinical characteristics, and precisely locate the urethral opening. These aspects were determined by experienced pediatric urologists during a pre-surgery physical examination, along with a questionnaire filled out by us in collaboration with the child’s parents.
The control group was recruited from the same healthcare institutions where the hypospadias cases were collected. These were male children who were visiting for conditions unrelated to hypospadias or any genital system anomalies. A pediatric specialist conducted a thorough clinical examination to confirm that they had no personal or familial history of hypospadias and were free from other external genital deformities, including cryptorchidism, micropenis, penile curvature, or inguinal hernia.
Informed consent was obtained from the parents or legal guardians of all participants. The case-control study received approval from the Ethical Scientific Committee of the Faculty of Natural and Life Sciences, University of Constantine 1. All procedures were carried out in compliance with the World Medical Association’s 1989 Declaration of Helsinki, which served as the protocol’s guide.
Genetic analysis
Blood sampling and DNA extraction:
Ten mL of venous blood were collected in tubes containing ethylenediaminotetracetic acid (EDTA). Genomic DNA from peripheral leukocytes was isolated using the sodium chloride (NaCl) technique.18 The purity of the DNA was verified using a nanodrop (NanoDrop 2000, Thermo, Massachusetts, U.S.A.). The isolated DNA was diluted to 20ng/μL and preserved at -20 °C.
MTHFR C677T genotyping: The MTHFR C677T variant was genotyped using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The primer sequences used were: forward: 5’-TGAAGGAGAAGGTGTCTGCGGGA-3’ and reverse: 5’-AGGACGGTGCGGTGAGAGTG-3’. The PCR reaction mixture (20 μL) comprised template DNA, MgCl2 (50mM), deoxynucleotide triphosphate (dNTPs) mix (final concentration 0.2 mmol/L), oligonucleotide primers (100 ng/μL), and Taq DNA polymerase (5 U/μL). An Eppendorf Mastercycler was used for the amplification process, following these conditions: initial denaturation at 94 °C for 5 minutes, then 35 cycles of denaturation at 94 °C for 30 seconds, hybridization at 65 °C for 30 seconds, elongation at 72 °C for 30 seconds, and a final extension at 72 °C for 10 minutes.
The resulting PCR product (10 μL) was digested using the Hinf1 restriction enzyme (New England Biolabs). The fragmented products were separated on a 3% agarose gel and observed under ultraviolet light.
To ensure the accuracy of genotyping, all samples were analyzed in duplicate, and both positive and negative control samples with known genotypes were included in each PCR-RFLP run.
Statistics
Statistical analysis of the data was performed using SPSS software (version 26; SPSS Inc., Chicago, IL, U.S.A.). Continuous variables are presented as mean ± standard deviation (SD), while categorical variables are shown as frequencies and percentages. Baseline characteristics, genotype, and allele distribution were compared between case children and controls using chi-square or t-tests.
The chi-square test for goodness of fit was employed to assess if the genotype distributions within the two groups conformed to the expectations of Hardy-Weinberg equilibrium (HWE). Risk-factor analysis was conducted using logistic regression analysis. Results were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs). Statistical significance was determined at p<0.05.
Results
Characteristics of the study population
The characteristics of 105 patients with hypospadias and 125 controls are summarized in Table I. The mean age in the hypospadias group and the control group was 4.58 ± 2.52 and 4.39 ± 2.45 years, respectively. Isolated hypospadias was observed in 55.24% of patients.
| *Age given as mean ± standard deviation; others as n (%). **p < 0.05; CI, confidence interval; OR, odds ratio; t, Student’s t; χ2, chi-square. | |||||
| Table I. Comparison of the case and the control group’s baseline characteristics. | |||||
| Characteristics |
|
|
|
|
|
| Age (years) |
|
|
|
|
|
| Intrauterinegrowth restrection |
|
|
|
|
|
| Low birth weight (<2500 g) |
|
|
|
|
|
| Preterm birth (<37 weeks) |
|
|
|
|
|
| Advanced maternal age (>35 years at pregnancy) |
|
|
|
|
|
| Gestational hypertension |
|
|
|
|
|
| Gestational diabetes |
|
|
|
|
|
| Birth order (1st) |
|
|
|
|
|
| Consanguinity |
|
|
|
|
|
| Residence (rural) |
|
|
|
|
|
| Type of hypospadias |
|
|
|
|
|
| Anterior |
|
||||
| Middle |
|
||||
| Posterior |
|
||||
Significant differences were observed between the hypospadias and control groups regarding IUGR (p = 0.004, OR = 11.21, 95% CI: 1.45–16.50), gestational hypertension (p = 0.004, OR = 4.61, 95% CI: 1.52–13.94), and rural residence (p = 0.039, OR = 1.97, 95% CI: 1.03–3.78).
Additionally, the distribution of hypospadias types among patients indicated that anterior hypospadias was the most frequent (59 cases, 56.2%), followed by posterior hypospadias (32 cases, 30.5%) and middle hypospadias (14 cases, 5.2%).
MTHFR C677T variant genotype and allele frequencies
Genotyping results for the C677T locus within the MTHFR gene, determined by electrophoresis, are illustrated in Supplementary Figure S1. Three different genotypes were observed: one band (198 bp), two bands (198 and 175 bp), and one band (175 bp) in the wild-type homozygote (CC), heterozygote (CT), and mutant homozygote (TT). The presence of allele T corresponds to the presence of a cutting site for HinfI.
The MTHFR gene variant’s distribution did not follow the HWE in cases and control groups (p<0.05). The distribution of genotype and allele frequency of the MTHFR C677T variant in patients with hypospadias and healthy controls is presented in Table II. The genotype frequencies of the MTHFR C677T variant among patients were 44.76% for CC, 35.24% for CT, and 20% for TT. In the control group, the corresponding frequencies were 61.6% (CC), 25.6% (CT), and 12.8% (TT). Despite these variations, the MTHFR C677T variant’s genotype distribution was statistically significant between the group of patients with hypospadias and the control group under codominant genetic model CT vs. CC and TT vs CC (p=0.034, OR: 1.89, 95% CI: 1.04-3.44 and p=0.042, OR: 2.15, 95% CI: 1.02-4.53, respectively). Also, significance was noted when the controls and patients were compared using the dominant model CC vs. CT+TT (p=0.010, OR: 1.98, 95% CI: 1.17-3.35). Concerning allele distribution, patients’ T allele frequency was higher than controls (37.62% and 25.6%, respectively). The MTHFR C677T variant’s allele frequency differed significantly (p=0.005, OR: 1.75, 95% CI: 1.18-2.61).
| *p < 0.05; CI, confidence interval; OR, odds ratio; Ref, reference. | ||||
| Table II. Distribution of the MTHFR C677T genotype in cases and controls, n (%). | ||||
| Genetic model |
|
|
|
|
| Codominance | ||||
| CC |
|
|
|
|
| CT |
|
|
|
|
| TT |
|
|
|
|
| Dominance | ||||
| CC vs CT+TT |
|
|
|
|
| Recessive | ||||
| CC+CT vs TT |
|
|
|
|
| Alleles | ||||
| C |
|
|
|
|
| T |
|
|
|
|
Patient’s characteristics by methylene tetrahydrofolate reductase C677T gene variant in children with hypospadias
Table III summarizes data on various patients’ characteristics according to different MTHFR C677T genotypes (CC, CT, TT, and CT or TT). No significant variations were detected in age, residence, birth weight, hypertensive gestation, gestational diabetes, and birth order (all p> 0.05) among these genotypes. However, substantial distinctions were evident in IUGR, which demonstrated a significant association with the genotypes (p = 0.021).
| *p < 0.05; CI, confidence interval; OR, odds ratio; P1, comparison between three genotypes; P2, comparison between CT+TT vs CC. | |||||||
| Table III. Patient characteristics of children with hypospadias according to the MTHFR C677T gene variation. | |||||||
|
|
|||||||
|
|
|
|
|
|
|
||
| Age | <5 yr |
|
|
|
|
|
|
| >5 yr |
|
|
|
|
|||
| Intrauterine growth restriction | Yes |
|
|
|
|
|
|
| No |
|
|
|
|
|||
| Preterm birth | Yes |
|
|
|
|
|
|
| No |
|
|
|
|
|||
| Birth weight | <2500 g |
|
|
|
|
|
|
| >2500 g |
|
|
|
|
|||
| Maternal age | <35 yr |
|
|
|
|
|
|
| >35 yr |
|
|
|
|
|||
| Gestational hypertension | Yes |
|
|
|
|
|
|
| No |
|
|
|
|
|||
| Gestational diabetes | Yes |
|
|
|
|
|
|
| No |
|
|
|
|
|||
| Birth order | 1 |
|
|
|
|
|
|
| >1 |
|
|
|
|
|||
| Consanguinity | Yes |
|
|
|
|
|
|
| No |
|
|
|
|
|||
| Residence | Rural |
|
|
|
|
|
|
| Urban |
|
|
|
|
|||
| Isolated hypospadias | Yes |
|
|
|
|
|
|
| No |
|
|
|
|
|||
Association between maternal periconceptional folic acid supplement intake, anatomical types of hypospadias and MTHFR C677T genotypes
Supplementary Table S1 examines the relationship between maternal periconceptional folic acid supplement intake, anatomical types of hypospadias, and the MTHFR C677T genotypes (CC, CT, TT). A statistically significant association was found between the MTHFR C677T genotypes and the anatomical types of hypospadias when folic acid was taken (p = 0.006).
Association between maternal periconceptional folic acid supplement intake, isolated/non-isolated hypospadias and MTHFR C677T genotypes
An association between maternal periconceptional folic acid supplement intake, isolated/non-isolated hypospadias, and MTHFR C677T genotypes was analyzed. A significant association was observed with the TT genotype in isolated cases (p = 0.038, OR = 3.47 with a 95% CI of 1.03-11.68), suggesting a potential role of folic acid in modifying hypospadias risk. However, other genotypes and non-isolated cases did not show consistent significant associations (Supplementary Table S2).
Multivariate logistic regression
The results of multiple logistic regression analyses (Table IV) were used to identify the best independent predictors. In this analysis, IUGR (p = 0.030, OR = 10.07, 95% CI: 1.26-80.49), gestational hypertension (p = 0.016, OR = 4.27, 95% CI: 1.31-13.89), rural residency (p = 0.040, OR = 1.97, 95% CI: 1.03-13.89) and MTHFR C677T variant (p = 0.045, OR = 2.15, 95% CI: 1.01-4.53) were found to be independent potential risk factors for hypospadias development.
| *p < 0.05; CI, confidence interval; OR, odds ratio. | ||
| Table IV. Logistic regression analysis of linked risk factors for patients with hypospadias. | ||
| Risk factor |
|
|
| Preterm birth |
|
|
| Birth weight (<2500 g) |
|
|
| Intrauterine growth restriction |
|
|
| Maternal age (> 35 years) |
|
|
| Gestational diabetes |
|
|
| Gestational hypertension |
|
|
| Consanguinity |
|
|
| Maternal periconceptional folic acid supplement intake |
|
|
| Residence (rural) |
|
|
| MTHFR C677T variant |
|
|
Discussion
Hypospadias, a common congenital anomaly affecting the male external genitalia, is believed to arise from a complex interplay of genetic and environmental factors, presenting a diverse range of treatment approaches.19 This study aimed to explore the potential association between the C677T variant of the MTHFR gene, maternal intake of folic acid supplements during the periconceptional period, and susceptibility to hypospadias in Algerian children. Our findings suggest a significant association between this genetic variant and the risk of developing hypospadias, which could have important implications for the etiological understanding and clinical management of this congenital anomaly.
Investigations into MTHFR gene variants and their association with congenital genital anomalies, such as hypospadias, are currently limited. The MTHFR gene is crucial in folate metabolism, essential for DNA synthesis and methylation, and influencing cellular division and development.20 The C677T variant alters the enzyme’s structure, reducing its activity, leading to hyperhomocysteinemia, and reducing the availability of active folate necessary for converting homocysteine to methionine. Methionine serves as a precursor for S-adenosylmethionine (SAM), a universal methyl donor in various methylation reactions, including DNA methylation.21 Disruptions in DNA methylation, an epigenetic mechanism, are associated with various developmental disorders, including congenital anomalies.22 Our study’s findings suggest that the C677T variant of the MTHFR gene, through its effects on enzymatic activity and increased homocysteine levels, may disrupt critical DNA methylation processes essential for normal embryonic development. This disruption could potentially affect signaling pathways and crucial genes involved in the formation of male genital organs, thereby contributing to the development of hypospadias.
In our study, the violation of HWE in both cases and controls could be attributed to several factors. First, the Algerian population may present genetic heterogeneity due to substructure, which could lead to slight deviations from HWE.23,24 Additionally, given the relatively small sample size, minor violations are not uncommon due to sampling variability.25,26 Notably, the violation of HWE in cases may reflect a genetic predisposition to certain genetic diseases, as affected individuals are often more likely to carry specific genotypes that deviate from equilibrium. These deviations can indicate natural selection or disease-related factors, where certain alleles confer an increased risk or resistance, altering genotype frequencies compared to what is expected in an equilibrium population.27 Furthermore, we ensured the accuracy of our genotyping by including duplicates and control samples, which suggests that this deviation is more likely due to population or disease-specific factors rather than technical errors.
Our findings revealed a prevalence of 44.76% CC genotype among patients, dominating the genetic profiles, whereas controls showed a dominant 61.6% CC genotype, with 35.24% CT and 20% TT among patients and 25.6% CT and 12.8% TT among controls. Importantly, the presence of the 677T allele was notably higher in patients with hypospadias (37.62%) compared to controls (25.6%). Our study highlighted notable variations between patients and controls’ genotype distribution and allele frequency (p=0.034, p=0.042, p=0.005, respectively; Table II).
These results contrast with Dokter et al.’s16 large-scale study in the Netherlands, which included over 800 cases and found no significant association between the MTHFR C677T variant and hypospadias. This discrepancy may be attributed to differences in study populations, as genetic and environmental factors vary between regions. The Algerian population may have distinct genetic predispositions or environmental exposures influencing the role of the MTHFR C677T variant in hypospadias risk. Additionally, while Dokter et al.16 analyzed a much larger cohort, our study provides an initial exploration within a North African population, emphasizing the need for further regional studies to confirm these findings.
A statistically significant correlation emerged in comparing patients and controls based on CC genotype versus CT+TT genotype (p=0.010). Our study represents the first exploration of the potential correlation between the MTHFR C667T variant and hypospadias risk in Algerian children, marking a significant discovery of an association between the MTHFR C677T variant and hypospadias.
However, the impact of the MTHFR C677T gene variant extends beyond hypospadias to various health conditions, including impaired renal function and urinary tract anomalies. This variant, associated with elevated homocysteine levels, may contribute to renal impairment in young people with high blood pressure and expectant mothers with preeclampsia.28 Additionally, it may affect renal function in pregnant women with preeclampsia, potentially leading to increased urinary protein levels.29 Studies have also highlighted a significant link between MTHFR C677T and urinary tract anomalies in girls, indicating a potential association between this gene variant and diverse urogenital development across genders.30
In our cases, we observed a significant correlation between IUGR and the MTHFR C677T variant (p=0.021; Table III). These findings align with multiple studies; for instance, Alset et al.31 identified a connection between MTHFR C677T and higher risks of fetal growth impairment and susceptibility to IUGR. Global populations exhibit a connection between the MTHFR C677T change and a higher risk of IUGR, as reported in a systematic review and meta-analysis.32 Furthermore, studies33 have demonstrated the significance of the C677T (Ala222Val) variant of the MTHFR gene in the development of IUGR.
Additionally, IUGR remained significantly associated with hypospadias in logistic regression (p=0.030, OR: 10.07, 95% CI: 1.26-23.49). Multiple studies have investigated the potential link between IUGR and hypospadias.34,35 The findings suggest a higher incidence of hypospadias among male infants when birth weight is below the 10th percentile and growth restriction is present, compared to those with normal birth weights. Moreover, IUGR has been linked to placental abnormalities, such as maternal vascular malperfusion, potentially contributing to hypospadias development.
Gestational hypertension and rural residence remained significantly associated with hypospadias in logistic regression (p=0.016, OR: 4.27, 95% CI: 1.31-13.89 and p=0.040, OR: 1.97, 95% CI: 1.03-3.78; respectively). Our findings align with Greenhill et al and Sherriff et al, indicating that maternal hypertensive disorders have been connected to an elevated risk of hypopadias in offspring.36,37 Additionally, regarding rural residence as a potential risk factor for hypospadias, our findings were consistent with Moustafa et al.38, who found that rural residence was one of the most independent predictors for hypospadias.
The significant association between gestational hypertension and hypospadias suggests that hypertensive complications could create an unfavorable intrauterine environment, influencing genital development. Additionally, rural residence, which is associated with potentially increased exposure to environmental factors or limited prenatal care, could also play a role in the increased risk.
Our study identifies, for the first time, that the MTHFR C677T presents a risk factor for hypospadias (p=0.045, OR: 2.15, 95% CI: 1.01-4.53). This underscores a significant correlation between a higher chance of developing hypospadias and the MTHFR gene’s C677T variation. These findings suggest a strong link between this variant and the studied risk. However, further investigations are required to validate this association and to elucidate the underlying mechanisms influenced by the MTHFR gene’s C677T variation.
Our study examined the association between maternal intake of folic acid supplements during the periconceptional period, anatomical types of hypospadias, and MTHFR C677T genotypes, as presented in Supplementary Table S1. The results indicate a statistically significant association between MTHFR C677T genotypes and anatomical types of hypospadias when folic acid is taken (p = 0.006). These findings suggest that the interaction between MTHFR C677T genotype and folic acid intake may play a role in determining specific anatomical types of hypospadias observed. Specifically, children with certain genotypes (e.g., CT and TT) may have a differential risk of developing more severe forms of hypospadias depending on whether their mothers took folic acid supplements during the periconceptional period. According to a study by Dokter et al, infants from mothers who did not use folic acid supplements appeared to have an increased risk of midshaft hypospadias (OR 1.6; 95% CI: 1.1–2.4).16
Folic acid is essential for DNA methylation and other critical biological processes during embryonic development.6 The presence of the MTHFR C677T variant, which alters folate metabolism, could influence these processes in a way that modifies the risk of developing different forms of hypospadias. These results underscore the potential importance of folic acid as a modulating factor in the etiology of hypospadias and suggest that future studies should further explore this interaction to better understand the underlying mechanisms and develop more targeted prevention strategies.
Furthermore, the results in Supplementary Table S2 suggest a significant association between maternal periconceptional folic acid supplement intake, MTHFR C677T genotypes, and isolated types of hypospadias. Specifically, the TT genotype appears to be statistically significantly associated with isolated cases of hypospadias (p = 0.038, OR = 3.47 with a 95% confidence interval of 1.03-11.68), indicating a potential role of folic acid in modifying hypospadias risk. However, other genotypes and non-isolated cases do not show consistent significant associations.
These findings collectively suggest that folic acid may play a differential role depending on specific types of hypospadias, whether anatomical or isolated. This highlights the importance of future studies to better understand this interaction and its clinical implications. Understanding the role of folic acid in relation to the MTHFR C677T genotype could lead to improved strategies for the prevention and management of hypospadias, particularly in populations with a higher prevalence of this genetic variant.
This study has some limitations that should be considered. The small sample size may affect the generalizability of our findings and could also contribute to the deviation from HWE observed in the case and control groups. This deviation may reflect undetected population substructure or selection bias strict inclusion criteria. Although all included patients had a confirmed 46,XY karyotype and syndromic features or ambiguous genitalia were excluded, no systematic hormonal or molecular genetic testing was conducted to identify other potential endocrine or monogenic causes of hypospadias. The absence of such investigations is mainly due to the retrospective design of the study and limited available resources. Consequently, the differentiation between isolated nonsyndromic hypospadias and cases potentially associated with subtle endocrine or genetic abnormalities may be incomplete. In addition, maternal DNA was not available for analysis, and thus maternal MTHFR genotype information was not assessed, despite evidence suggesting that maternal gene variants and folic acid deficiency may be associated with miscarriages and congenital anomalies. Additionally, the definition of rural and urban residency was based only on maternal residence during pregnancy, without considering environmental exposures. Despite these limitations, our study provides preliminary insights into the genetic and environmental factors of hypospadias in Algeria. Larger, prospective, and multicenter studies with more comprehensive endocrine and genetic assessments are needed to confirm these findings and better elucidate gene-environment interactions.
In conclusion, our study is the first to demonstrate an association between the C677T variant of the MTHFR gene and hypospadias in the Algerian population. These findings underscore the importance of genetics in the etiology of hypospadias and suggest that genetic screening could become a valuable tool for identifying at-risk children. However, further studies with larger sample sizes and functional analyses are necessary to validate these results and elucidate the underlying biological mechanisms influenced by the MTHFR C677T variant.
Acknowledgements
The authors are grateful for the participation of the parents/legal guardians of both patients and controls. We extend our appreciation to Madam Yasmina Dadci, responsible for DNA extraction at the Biology and Genetics Laboratory, CHU Constantine, as well as the entire staff of the Pediatric Urology Unit, CHU Setif, and the Pediatric Surgery Department at the Specialized Mother and Child Hospital in El Mansourah, Constantine, Algeria, for their valuable contributions to this work.
Ethical approval
The study was approved by Ethical Scientific Committee of the Faculty of Natural and Life Sciences at the University of Constantine 1 (date: 28.11.2019, number: EC/UMC/SNV/02/11-2019).
Source of funding
The authors declare the study received no funding.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Ye ZH, Chen HS, Zhang ZC, Wang X, Liu X, Wei GH. Parental smoking and risk of hypospadias: an updated meta-analysis of observational studies. Front Pediatr 2023; 11: 1003037. https://doi.org/10.3389/fped.2023.1003037
- Shih EM, Graham JM. Review of genetic and environmental factors leading to hypospadias. Eur J Med Genet 2014; 57: 453-463. https://doi.org/10.1016/j.ejmg.2014.03.003
- Chang J, Wang S, Zheng Z. Etiology of hypospadias: a comparative review of genetic factors and developmental processes between human and animal models. Res Rep Urol 2020; 12: 673-686. https://doi.org/10.2147/RRU.S276141
- Halaseh SA, Halaseh S, Ashour M. Hypospadias: a comprehensive review including its embryology, etiology and surgical techniques. Cureus 2022; 14: e27544. https://doi.org/10.7759/cureus.27544
- Bouty A, Ayers KL, Pask A, Heloury Y, Sinclair AH. The genetic and environmental factors underlying hypospadias. Sex Dev 2015; 9: 239-259. https://doi.org/10.1159/000441988
- Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 2012; 3: 21-38. https://doi.org/10.3945/an.111.000992
- Li W, Li Z, Li S, Wang X, Wilson JX, Huang G. Periconceptional folic acid supplementation benefit to development of early sensory-motor function through increase DNA methylation in rat offspring. Nutrients 2018; 10: 292. https://doi.org/10.3390/nu10030292
- Dong J, Yin LL, Deng XD, et al. Initiation and duration of folic acid supplementation in preventing congenital malformations. BMC Med 2023; 21: 292. https://doi.org/10.1186/s12916-023-03000-8
- Hall J, Solehdin F. Folic acid for the prevention of congenital anomalies. Eur J Pediatr 1998; 157: 445-450. https://doi.org/10.1007/s004310050850
- Weiner AS, Boyarskikh UA, Voronina EN, Mishukova OV, Filipenko ML. Methylenetetrahydrofolate reductase C677T and methionine synthase A2756G polymorphisms influence on leukocyte genomic DNA methylation level. Gene 2014; 533: 168-172. https://doi.org/10.1016/j.gene.2013.09.098
- Sohn KJ, Jang H, Campan M, et al. The methylenetetrahydrofolate reductase C677T mutation induces cell-specific changes in genomic DNA methylation and uracil misincorporation: a possible molecular basis for the site-specific cancer risk modification. Int J Cancer 2009; 124: 1999-2005. https://doi.org/10.1002/ijc.24003
- de Arruda ITS, Persuhn DC, de Oliveira NFP. The MTHFR C677T polymorphism and global DNA methylation in oral epithelial cells. Genet Mol Biol 2013; 36: 490-493. https://doi.org/10.1590/S1415-47572013005000035
- Kim JO, Park HS, Ryu CS, et al. Interplay between 3’-UTR polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene and the risk of ischemic stroke. Sci Rep 2017; 7: 12464. https://doi.org/10.1038/s41598-017-12668-x
- Tabatabaei RS, Fatahi-Meibodi N, Meibodi B, et al. Association of fetal MTHFR C677T polymorphism with susceptibility to neural tube defects: a systematic review and update meta-analysis. Fetal Pediatr Pathol 2022; 41: 225-241. https://doi.org/10.1080/15513815.2020.1775734
- Rai V. Strong association of C677T polymorphism of methylenetetrahydrofolate reductase gene with nosyndromic cleft lip/palate (nsCL/P). Indian J Clin Biochem 2018; 33: 5-15. https://doi.org/10.1007/s12291-017-0673-2
- Dokter EM, van Rooij IA, Wijers CH, et al. Interaction between MTHFR 677C>T and periconceptional folic acid supplementation in the risk of Hypospadias. Birth Defects Res A Clin Mol Teratol 2016; 106: 275-284. https://doi.org/10.1002/bdra.23487
- Kon M, Suzuki E, Dung VC, et al. Molecular basis of non-syndromic hypospadias: systematic mutation screening and genome-wide copy-number analysis of 62 patients. Hum Reprod 2015; 30: 499-506. https://doi.org/10.1093/humrep/deu364
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215. https://doi.org/10.1093/nar/16.3.1215
- Gozar H, Bara Z, Dicu E, Derzsi Z. Current perspectives in hypospadias research: a scoping review of articles published in 2021 (Review). Exp Ther Med 2023; 25: 211. https://doi.org/10.3892/etm.2023.11910
- Raghubeer S, Matsha TE. Methylenetetrahydrofolate (MTHFR), the one-carbon cycle, and cardiovascular risks. Nutrients 2021; 13: 4562. https://doi.org/10.3390/nu13124562
- Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet 2015; 58: 1-10. https://doi.org/10.1016/j.ejmg.2014.10.004
- Vukic M, Daxinger L. DNA methylation in disease: immunodeficiency, centromeric instability, facial anomalies syndrome. Essays Biochem 2019; 63: 773-783. https://doi.org/10.1042/EBC20190035
- Lucas-Sánchez M, Abdeli A, Bekada A, Calafell F, Benhassine T, Comas D. The impact of recent demography on functional genetic variation in North African human groups. Mol Biol Evol 2024; 41: msad283. https://doi.org/10.1093/molbev/msad283
- Vilà-Valls L, Abdeli A, Lucas-Sánchez M, et al. Understanding the genomic heterogeneity of North African Imazighen: from broad to microgeographical perspectives. Sci Rep 2024; 14: 9979. https://doi.org/10.1038/s41598-024-60568-8
- Cao Y, Chen RC, Katz AJ. Why is a small sample size not enough? Oncologist 2024; 29: 761-763. https://doi.org/10.1093/oncolo/oyae162
- Tipton E, Hallberg K, Hedges LV, Chan W. Implications of small samples for generalization: adjustments and rules of thumb. Eval Rev 2017; 41: 472-505. https://doi.org/10.1177/0193841X16655665
- Salanti G, Amountza G, Ntzani EE, Ioannidis JP. Hardy-Weinberg equilibrium in genetic association studies: an empirical evaluation of reporting, deviations, and power. Eur J Hum Genet 2005; 13: 840-848. https://doi.org/10.1038/sj.ejhg.5201410
- Elsaid HH, El-Hefnawy KA, Elalawi SM. C677T MTHFR Gene polymorphism is contributing factor in development of renal impairment in young hypertensive patients. Indian J Clin Biochem 2021; 36: 213-220. https://doi.org/10.1007/s12291-020-00890-w
- Yun L, Ge M, Xu R, Zheng F, Zhao X, Li X. C677T Gene polymorphism of MTHFR is a risk factor for impaired renal function in pregnant women with preeclampsia in the Chinese Han population. Front Cardiovasc Med 2022; 9: 902346. https://doi.org/10.3389/fcvm.2022.902346
- Behunova J, Klimcakova L, Podracka L. Urinary tract anomalies associated with MTHFR gene polymorphism C677T in girls. Kidney Blood Press Res 2011; 34: 465-471. https://doi.org/10.1159/000329935
- Alset D, Kubyshkina DV, Butenko EV, Pokudina IO, Shkurat TP. Association of C677T and A1298C genetic polymorphisms in MTHFR gene with fetal growth restriction, small for gestational age and low birth weight: a meta-analysis. Hum Gene 2023; 37: 201190. https://doi.org/10.1016/j.humgen.2023.201190
- Bahrami R, Schwartz DA, Asadian F, et al. Association of MTHFR 677C>T polymorphism with IUGR and placental abruption risk: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2021; 256: 130-139. https://doi.org/10.1016/j.ejogrb.2020.11.016
- Tyutyunnik VL, Kan NE, Mantrova DA, Lomova NA, Klimantsev IV, Donnikov AE. The role of methylentetrahydrofolate reductase (MTHFR) gene polymorphism in intrauterine growth restriction development. Obstet Gynecol 2018; 12: 23-28. https://doi.org/10.18565/aig.2018.12.23-28
- Toufaily MH, Roberts DJ, Westgate MN, Hunt AT, Holmes LB. Hypospadias, intrauterine growth restriction, and abnormalities of the placenta. Birth Defects Res 2018; 110: 122-127. https://doi.org/10.1002/bdr2.1087
- Chen MJ, Macias CG, Gunn SK, et al. Intrauterine growth restriction and hypospadias: is there a connection? Int J Pediatr Endocrinol 2014; 2014: 20. https://doi.org/10.1186/1687-9856-2014-20
- Greenhill C. Development: Hypospadias linked with maternal hypertension. Nat Rev Endocrinol 2014; 10: 189. https://doi.org/10.1038/nrendo.2014.5
- Sheriff FR, Lopez A, Lupo PJ, Seth A, Jorgez C, Agopian AJ. Maternal hypertension and hypospadias in offspring: a systematic review and meta-analysis. Birth Defects Res 2019; 111: 9-15. https://doi.org/10.1002/bdr2.1415
- Moustafa W, Abouelella S, Tawfik M, Abuelyazeed M, Zanaty F. Genetic and other epidemiological risk factors of infants and children with hypospadias: a case control study. Afr J Urol 2023; 29: 55. https://doi.org/10.1186/s12301-023-00386-y
Copyright and license
Copyright © 2025 The Author(s). This is an open access article distributed under the Creative Commons Attribution License (CC BY), which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is properly cited.















